Acids Bases and p H Acids and Bases
Acids, Bases and p. H
Acids and Bases n used for different functions in body (such as digestion). n p. H is different in different parts of body, like acid (p. H 3) in stomach and basic (p. H 8) in small intestines
1. Acids n Any compound that GIVES OFF H+ ions in solution n n Ex. HCl Traits: Add water H+ and Cl- Sour n ex. Juices, vinegar, HCl n

2. Bases n Any compound that GIVES OFF OHions in solution n n Ex. Na. OH Add water Na+ and OH- Traits: n Slippery n Ex. Soaps, detergent, ammonia
3. Neutralization n Equal amounts of acid and base together Acid (H+) + Base (OH-) HOH = n n H 2 O Acids and Bases make salts when you have same amounts Ex. HCl (Hydrochloric acid) H 2 O (water) + + Na. OH (Sodium hydroxide) Na. Cl (table salt)
4. BUFFERS n n substances that react with strong acids or bases to prevent sudden p. H change What do you take when stomach is upset? ? (HINT: What digestive factor is in the stomach? ? ) n Antacids (made of calcium carbonate…a weak base!) ie. Tums or M. O. M
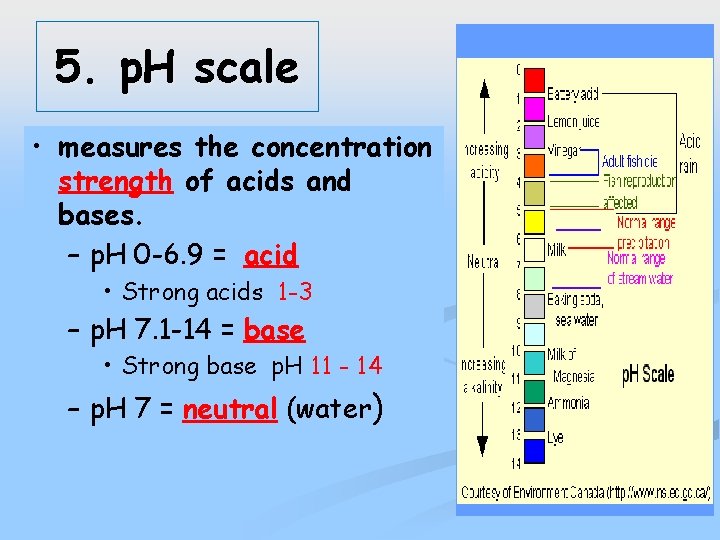
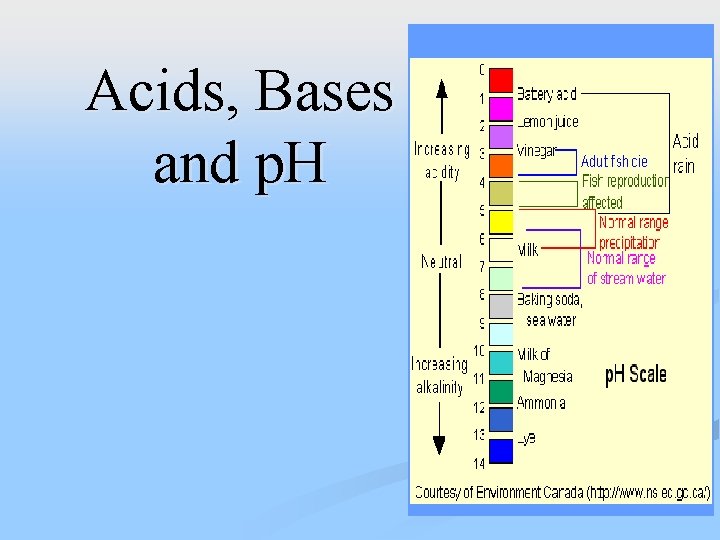
5. p. H scale • measures the concentration strength of acids and bases. – p. H 0 -6. 9 = acid • Strong acids 1 -3 – p. H 7. 1 -14 = base • Strong base p. H 11 - 14 – p. H 7 = neutral (water)

6. Litmus paper • Tests for Acids or bases • Turns Red = acidic • Turns Blue = basic

Let’s Practice ……p. H activity YOU TRY: 1. Indicator test 2. Watch VIDEO and complete quiz: (3: 49) http: //www. brainpop. com/science/matterandchemistry/phsc ale/ 3. Virtual Lab What is the p. H of common solutions? n http: //glencoe. mheducation. com/sites/0078600472/student_v iew 0/unit 6/chapter 21/virtual_lab. html
- Slides: 9