Acids Bases and Indicators What are Acids Common

Acids, Bases and Indicators

What are Acids? � Common substances used in everyday life � Some occur naturally & some are synthetic � Some are dangerous because they are corrosive (they can ‘eat away’ metal, wood and clothing and burn your skin). � Acids can be dilute (contains a large amount of water) or concentrated (must be handled with great care).
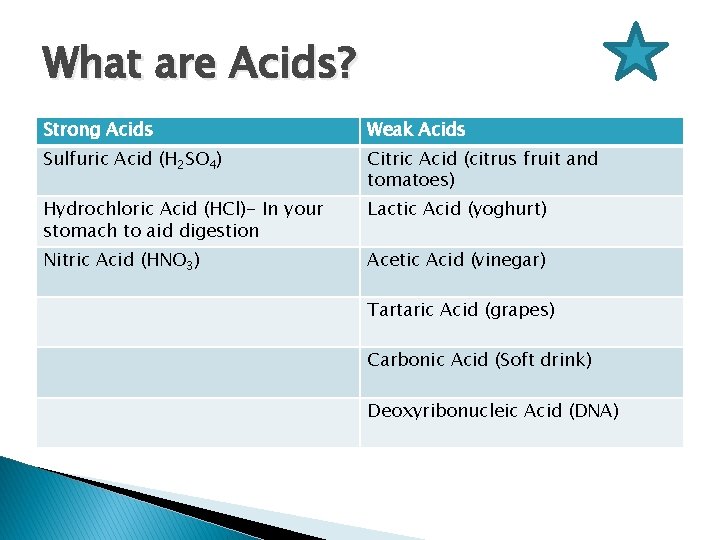
What are Acids? Strong Acids Weak Acids Sulfuric Acid (H 2 SO 4) Citric Acid (citrus fruit and tomatoes) Hydrochloric Acid (HCl)- In your stomach to aid digestion Lactic Acid (yoghurt) Nitric Acid (HNO 3) Acetic Acid (vinegar) Tartaric Acid (grapes) Carbonic Acid (Soft drink) Deoxyribonucleic Acid (DNA)

What are Bases? � Bases neutralise (cancel out) acids � E. g. toothpastes contain weak bases to neutralise acid on your teeth � Used to dissolved grease or dirt � Bases that are soluble in water are called alkalis � They feel soapy because they turn the oils on your skin into soap

Acids and Teeth � When you eat, food remains between your teeth � Bacteria in your mouth feed on this food � Bacteria produce weak acids as waste � These acids react with your teeth causing decay � The mixture of bits of food, bacteria, acids and saliva that stick to your teeth is called plaque

Acids and teeth � The top of the tooth is covered in enamel (hardest substance in the body) � The inside of the tooth is made of a softer substance called dentine � If the bacteria & acids cause the enamel to decay, the tooth can be damaged rapidly

Acids and Teeth � The best way of getting rid of plaque and food particles from your teeth is by brushing them � Toothpastes contain abrasives such as finely powdered chalk that help scrape food particles from your teeth � Some toothpastes are highly basic to neutralise acids of decaying food � These react with tooth enamel in young people’s teeth to form a substance that is more resistant to acid attack

Stomach Acid � Gastric juice is produced in your stomach to help to digest the food you eat � This gastric juice consists of dilute hydrochloric acid, enzymes and water � The hydrochloric acid helps to kill most microbes � It also helps the enzymes to work as they will only function in the presence of an acid � Enzymes break down the proteins in your food into amino acids which are needed for growth � You stomach is protected from the acid by a sticky fluid called mucus

Acids in Food and Drink � Baking powder and self-raising flour contain baking soda (sodium hydrogen carbonate) and an acid substance such a scream of tartar � When it becomes moist the baking soda reacts with the acid to form carbon dioxide gas � The gas is trapped in the mixture in the form of bubbles and causes the cake to rise in the oven � Acids are also used to preserve food by preventing bacteria growth, e. g. vinegar in pickles or sauces

Indicators �A quick way to tell if a solution is acidic, basic or neutral is to use an acid-base indicator � Indicators indicate when an acid or base is present by changing colour � Some indicators occur naturally in dyes in plants � E. g. litmus comes from lichens which grow on the bark of trees & on rocks � In an acidic solution litmus turns red; and in a basic solution, it turns blue.
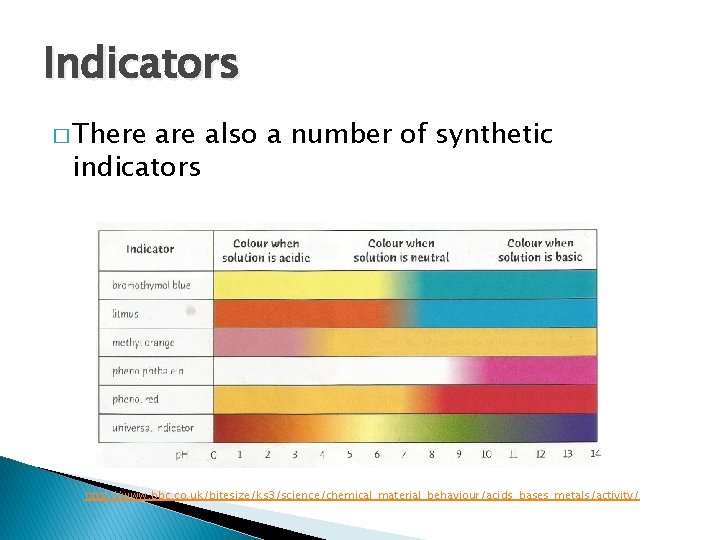
Indicators � There also a number of synthetic indicators http: //www. bbc. co. uk/bitesize/ks 3/science/chemical_material_behaviour/acids_bases_metals/activity/
- Slides: 11