Acids Bases Acids Acids are substances that are

Acids & Bases

Acids - Acids are substances that are corrosive

- Some acids are strong & are very corrosive, e. g. , hydrochloric acid (HCl), sulfuric acid (H 2 SO 4) - Some acids are weak & are not very corrosive, e. g. , citric acid (lemons), ethanoic acid (vinegar), carbonic acid (fizzy drinks)

- All acids have a sharp, sour taste - An acid is a substance that turns litmus red

Bases - Bases are substances that are corrosive - Some bases are strong & are very corrosive, e. g. , sodium hydroxide(Na. OH), calcium hydroxide(Ca(OH)2) - Some bases are weak & are not very corrosive, e. g. , toothpaste, oven cleaner, soap

- Bases are the opposite of acids - A base that is soluble in water is called an alkali - An alkali is a substance that turns litmus blue
Indicators - An indicator is a chemical which shows if a substance is acidic, alkaline or neutral by means of a colour change - Litmus is red in an acid & blue in an alkali (base) - Litmus cannot tell how strong or weak an acid or an alkali is
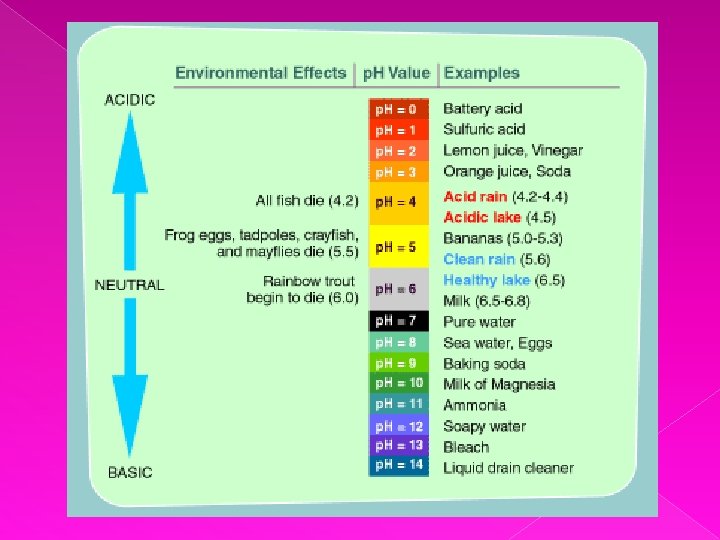
- The p. H scale is a scale that shows how acidic or alkaline a substance is - The scale goes from 0 – 14 - p. H < 7 = acidic p. H = 7 = neutral p. H > 7 = alkaline

- Strong acid = p. H close to 0 Weak acid = p. H close to 7 - Strong alkali = p. H close to 14 Weak alkali = p. H close to 7
- p. H is found using Universal indicator (paper or solution) - The indicator is added & a colour change occurs - The colour change is compared to a chart to find the p. H value

Neutralisation - When an acid & a base react together they form a salt & water - This is called a neutralistion reaction - A salt is formed when the metal ion in a base replaces the hydrogen ion in an acid
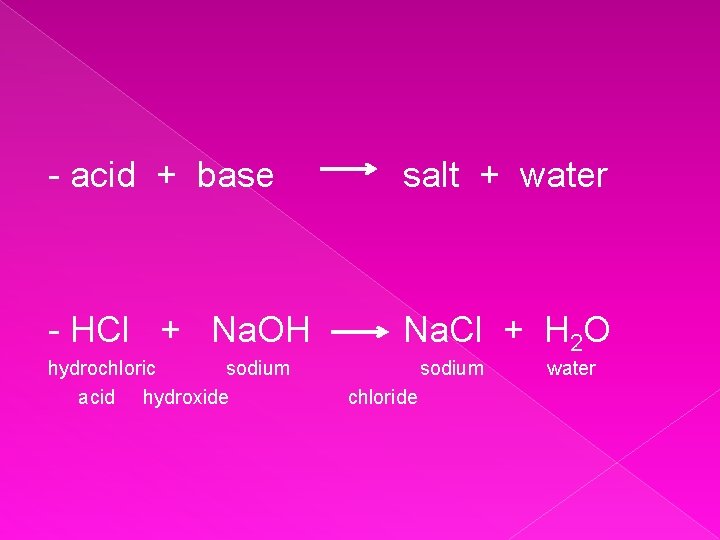
- acid + base salt + water - HCl + Na. OH Na. Cl + H 2 O hydrochloric sodium acid hydroxide sodium chloride water
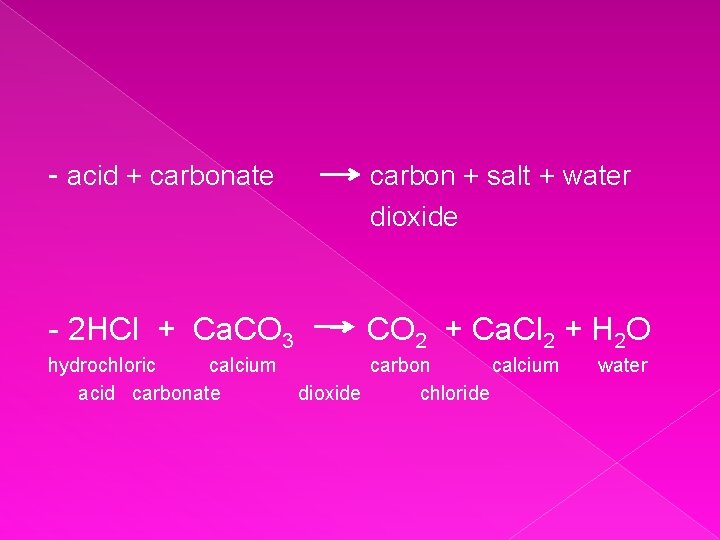
- acid + carbonate carbon + salt + water dioxide - 2 HCl + Ca. CO 3 CO 2 + Ca. Cl 2 + H 2 O hydrochloric calcium acid carbonate carbon calcium dioxide chloride water
- Slides: 14