Acids and Bases When a substance dissolves in

Acids and Bases When a substance dissolves in water it makes a solution. Solutions can be sorted by whether they are: acid, basic (alkali) or neutral.
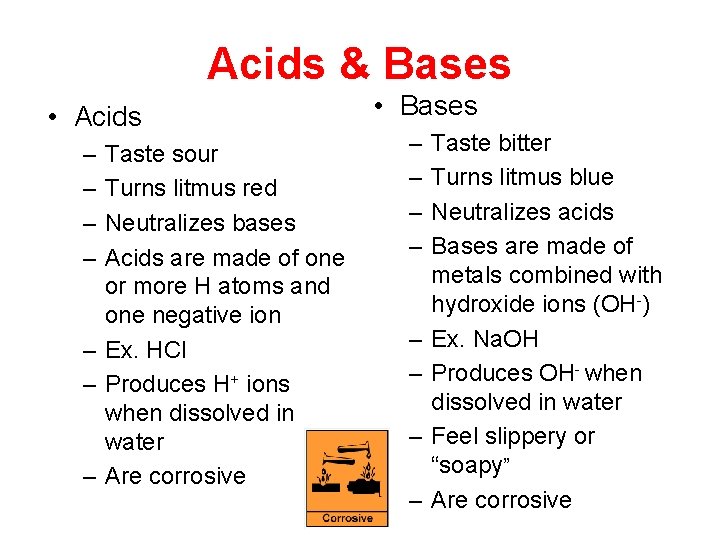
Acids & Bases • Acids – – Taste sour Turns litmus red Neutralizes bases Acids are made of one or more H atoms and one negative ion – Ex. HCl – Produces H+ ions when dissolved in water – Are corrosive • Bases – – – – Taste bitter Turns litmus blue Neutralizes acids Bases are made of metals combined with hydroxide ions (OH-) Ex. Na. OH Produces OH- when dissolved in water Feel slippery or “soapy” Are corrosive
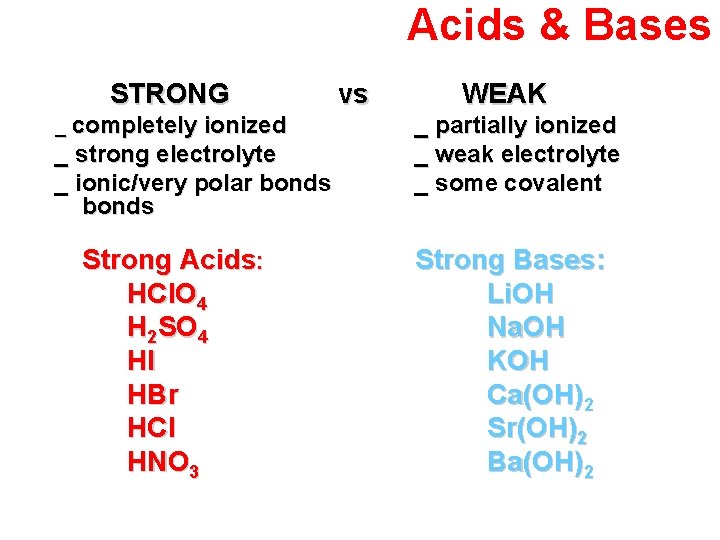
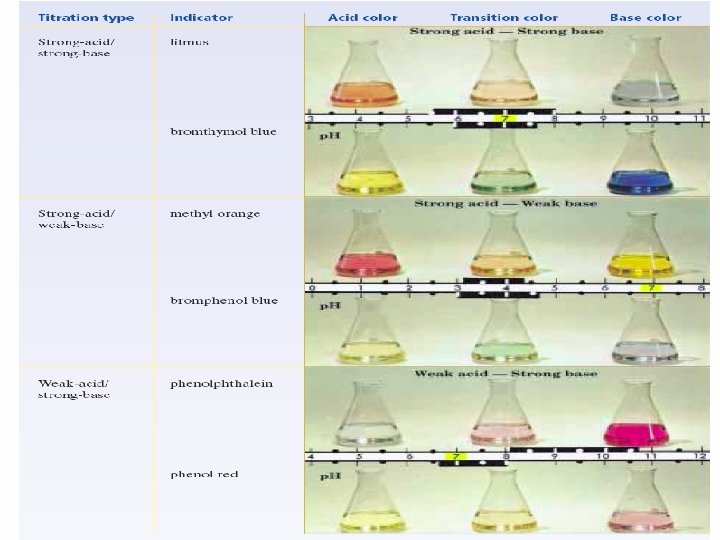
Acids & Bases STRONG _ completely ionized _ strong electrolyte _ ionic/very polar bonds Strong Acids: HCl. O 4 H 2 SO 4 HI HBr HCl HNO 3 vs WEAK _ partially ionized _ weak electrolyte _ some covalent Strong Bases: Li. OH Na. OH KOH Ca(OH)2 Sr(OH)2 Ba(OH)2

Acids There are many acids present in our everyday lives. Lemon juice contains citric acid, and vinegar contains acetic acid (also known as ethanoic acid). Some strong acids are hydrochloric acid, sulphuric acid and nitric acid. Some weak acids are ethanoic acid, citric acid and carbonic acid.

Bases Alkalis are present in many cleaning substances used in our homes. Kitchen cleaners are alkaline because they contain ammonia or sodium hydroxide, which attack grease. Calcium hydroxide and sodium hydroxide are strong alkalis. The most recognizable and common weak alkali is ammonia.
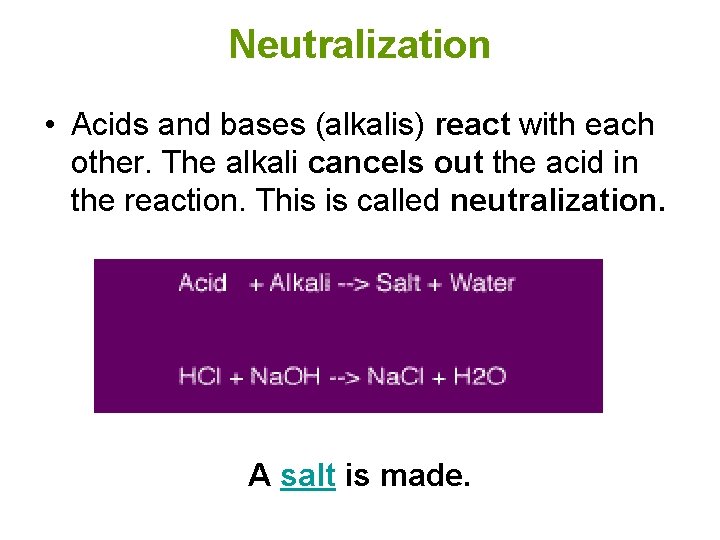
Neutralization • Acids and bases (alkalis) react with each other. The alkali cancels out the acid in the reaction. This is called neutralization. A salt is made.

Salts • The salt made depends on the acid and alkali used. • The salt contains the metal atom from the alkali, and the non-metal of the acid molecule. The salts of sulphuric acid are known as sulphates. The salts of hydrochloric acid are known as chlorides. The salts of nitric acid are known as nitrates.
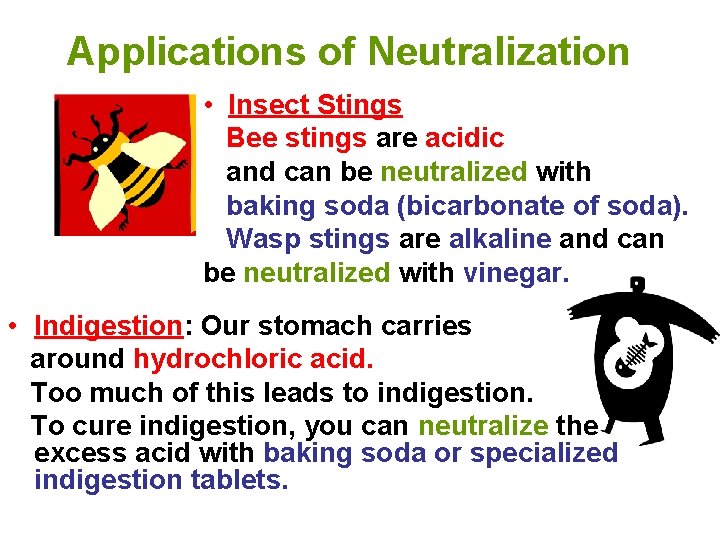
Applications of Neutralization • Insect Stings Bee stings are acidic and can be neutralized with baking soda (bicarbonate of soda). Wasp stings are alkaline and can be neutralized with vinegar. • Indigestion: Our stomach carries around hydrochloric acid. Too much of this leads to indigestion. To cure indigestion, you can neutralize the excess acid with baking soda or specialized indigestion tablets.

Soil Treatment: When soils are too acidic (often as a result of acid rain) they can be treated with slaked lime, chalk or quicklime, all alkalis. Plants and crops grow best in neutral soils. Factory Waste: Liquid waste from factories is often acidic. If it reaches a river it will destroy and kill sea life of many forms. Neutralizing the waste with slaked lime can prevent this.
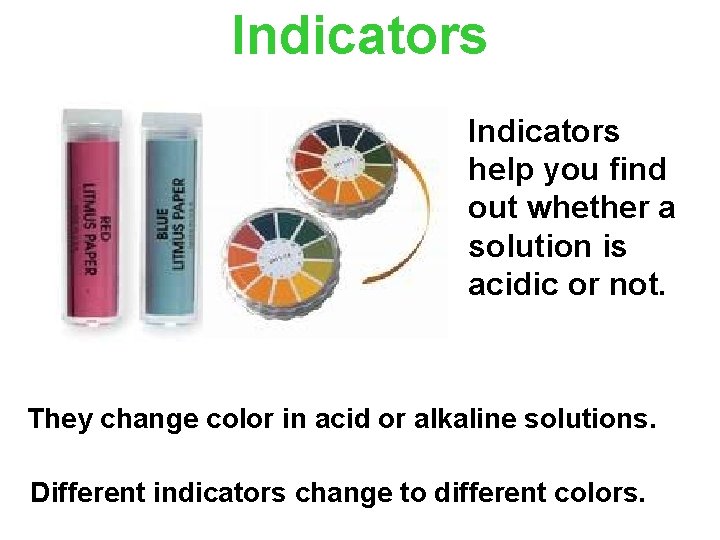
Indicators help you find out whether a solution is acidic or not. They change color in acid or alkaline solutions. Different indicators change to different colors.
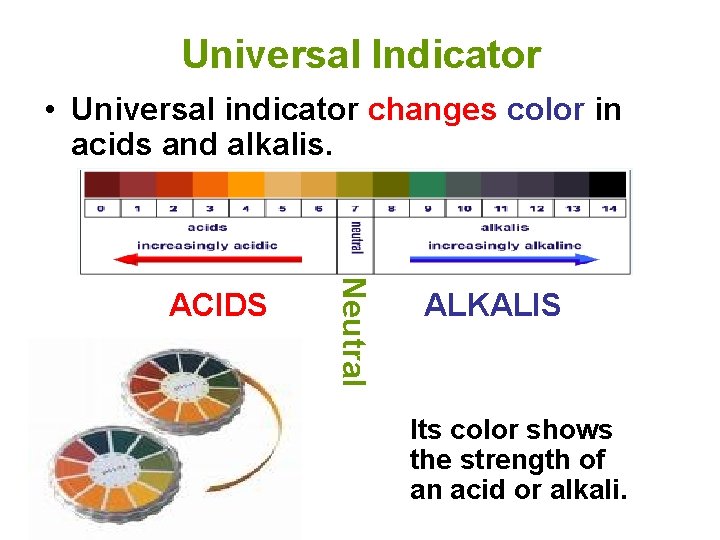
Universal Indicator • Universal indicator changes color in acids and alkalis. Neutral ACIDS ALKALIS Its color shows the strength of an acid or alkali.

Litmus Test • Litmus is an indicator. It changes color in acid and alkaline solutions. • Litmus is red in an acid. • Litmus is blue in an alkali.
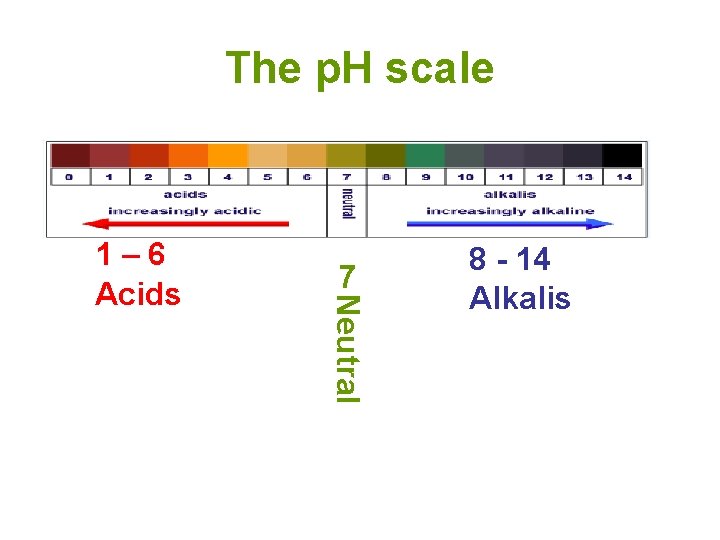
The p. H scale 7 Neutral 1– 6 Acids 8 - 14 Alkalis
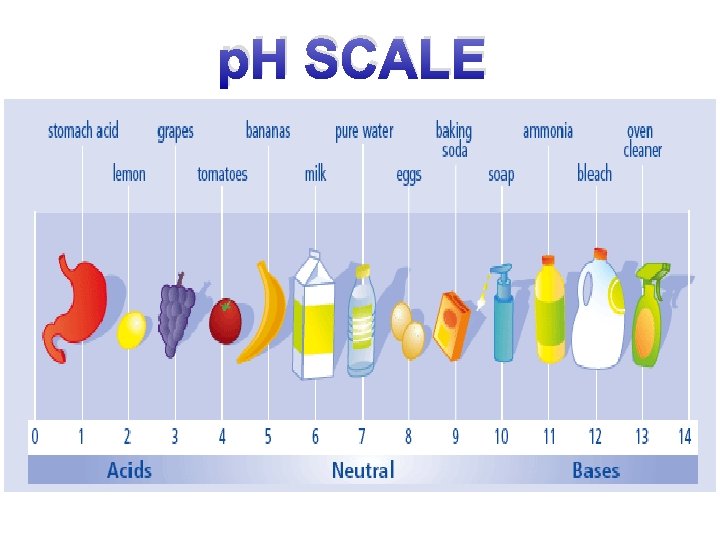
p. H SCALE
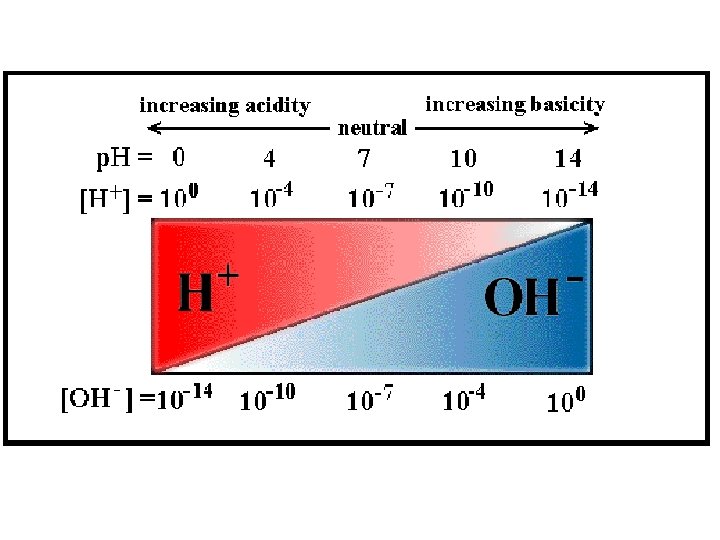
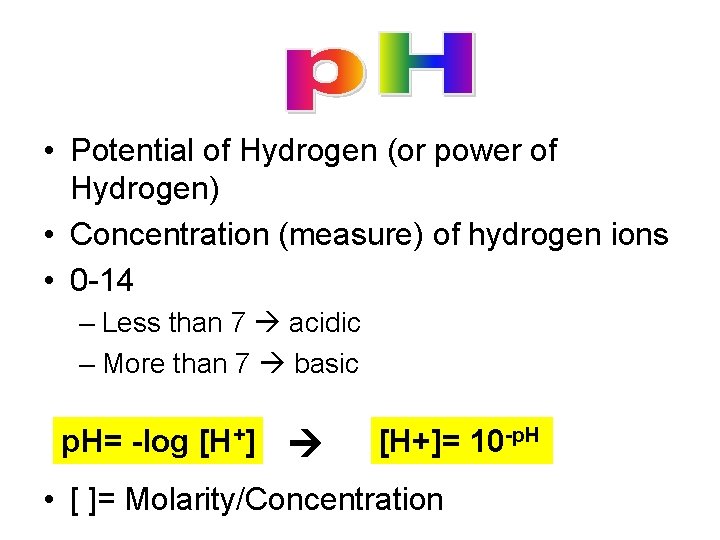
• Potential of Hydrogen (or power of Hydrogen) • Concentration (measure) of hydrogen ions • 0 -14 – Less than 7 acidic – More than 7 basic p. H= -log [H+]= 10 -p. H • [ ]= Molarity/Concentration
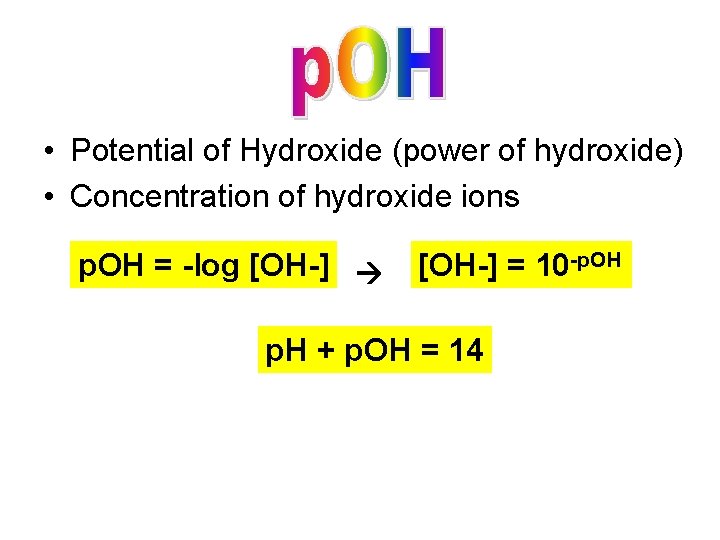
• Potential of Hydroxide (power of hydroxide) • Concentration of hydroxide ions p. OH = -log [OH-] = 10 -p. OH p. H + p. OH = 14
![p. H Calculations p. H = -log[H+] = 10 -p. H + p. OH p. H Calculations p. H = -log[H+] = 10 -p. H + p. OH](http://slidetodoc.com/presentation_image_h/1c507a385f2b5527a9777f91d1a2bfb1/image-19.jpg)
p. H Calculations p. H = -log[H+] = 10 -p. H + p. OH = 14 p. OH [H+] [OH-] = 1. 0 x 10 -14 p. OH = -log[OH-] = 10 -p. OH
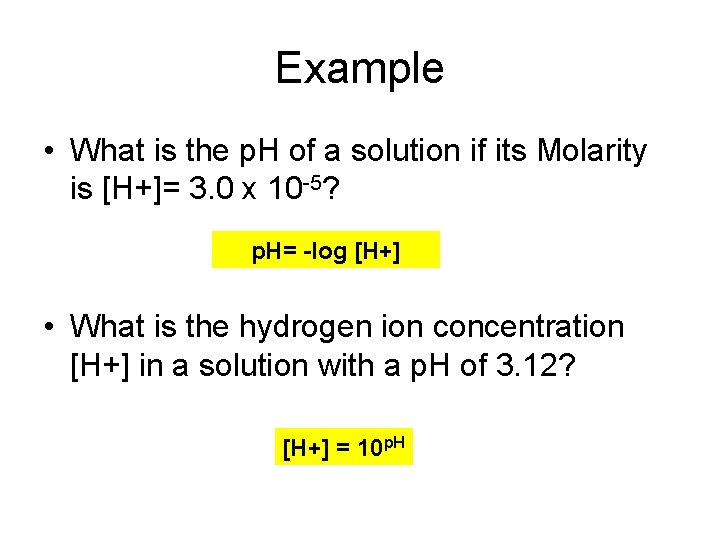
Example • What is the p. H of a solution if its Molarity is [H+]= 3. 0 x 10 -5? p. H= -log [H+] • What is the hydrogen ion concentration [H+] in a solution with a p. H of 3. 12? [H+] = 10 p. H
![Example • [ ]= Molarity/Concentration • What is the p. OH of a solution Example • [ ]= Molarity/Concentration • What is the p. OH of a solution](http://slidetodoc.com/presentation_image_h/1c507a385f2b5527a9777f91d1a2bfb1/image-21.jpg)
Example • [ ]= Molarity/Concentration • What is the p. OH of a solution that has a. 0658 moles of Na. OH in 0. 156 L of solution? M = moles/ L = [ ] p. OH = -log [OH-] • What is the [OH-] a solution p. H +ofp. OH = 14 if the p. H is 9? [OH-] = 10 p. OH
![Your turn…. What is the p. H of a solution if the [H+] is Your turn…. What is the p. H of a solution if the [H+] is](http://slidetodoc.com/presentation_image_h/1c507a385f2b5527a9777f91d1a2bfb1/image-22.jpg)
Your turn…. What is the p. H of a solution if the [H+] is 2. 8 x 10 -3? What is the [H+] of a solution if the p. H is 3. 5? What is the p. OH of Li. OH if you have 0. 45 moles in 0. 65 L? What is the [OH-] of a solution if the p. H is 5. 6? What is the p. H if the [OH-] is 4. 5 x 10 -2? p. H= -log [H+]= 10 p. H p. OH = -log [OH-] = 10 p. OH M = moles/ L = [ ] p. H + p. OH = 14
![“How to” guide to calculate Finding [H+] and [OH-] [H+] x [OH-] = 1 “How to” guide to calculate Finding [H+] and [OH-] [H+] x [OH-] = 1](http://slidetodoc.com/presentation_image_h/1c507a385f2b5527a9777f91d1a2bfb1/image-23.jpg)
“How to” guide to calculate Finding [H+] and [OH-] [H+] x [OH-] = 1 x 10 -14 M 2 [H+] = 1 x 10 -14 M 2 or [OH-] = 1 x 10 -14 M 2 [OH-] [H+] Finding p. H = -log [H+] Ex: find p. H if [H+] = 6. 70 x 10 -7 M In the calculator, enter : - Log 6. 70 exp – 7 = ans p. H = 6. 17
![continued How to find p. OH = -log [OH-] Example: What is the p. continued How to find p. OH = -log [OH-] Example: What is the p.](http://slidetodoc.com/presentation_image_h/1c507a385f2b5527a9777f91d1a2bfb1/image-24.jpg)
continued How to find p. OH = -log [OH-] Example: What is the p. OH of a solution that has a [H+] = 3. 0 x 10 -5 M First find [OH-]: [OH-] = 1 x 10 -14 M 2 3. 0 x 10 -5 M In the calculator, enter: - Log 3. 33 exp - 5 = ans. [OH-] = 3. 33 x 10 -10 M p. OH = 4. 48
![continued How to calculate [H+] from p. H Take the antilog of the negative continued How to calculate [H+] from p. H Take the antilog of the negative](http://slidetodoc.com/presentation_image_h/1c507a385f2b5527a9777f91d1a2bfb1/image-25.jpg)
continued How to calculate [H+] from p. H Take the antilog of the negative p. H Example: What is the [H+] of a solution with a p. H of 5. 25? In the calculator, enter: Shift log - 5. 25 = ans. [H+] = 5. 62 x 10 -6 M Last but not least, remember: p. H + p. OH = 14
- Slides: 25