Acids and Bases The Arrhenius concept Acid a
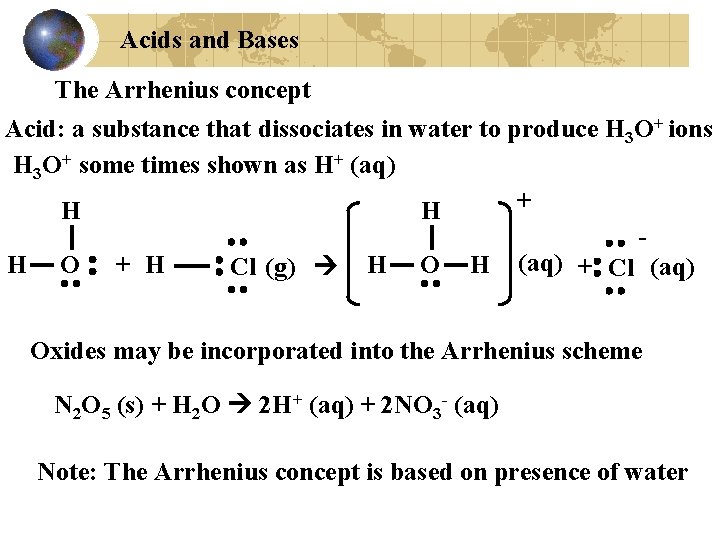
Acids and Bases The Arrhenius concept Acid: a substance that dissociates in water to produce H 3 O+ ions H 3 O+ some times shown as H+ (aq) + H H H O + H Cl (g) H O H (aq) + Cl (aq) Oxides may be incorporated into the Arrhenius scheme N 2 O 5 (s) + H 2 O 2 H+ (aq) + 2 NO 3 - (aq) Note: The Arrhenius concept is based on presence of water

The BrØnsted-Lowry Concept Acid : a substance that can donate a proton Base: a substance that can accept a proton CH 3 COOH (aq) + H 2 O (aq) H 3 O+ (aq) + CH 3 COO - (aq) CH 3 COOH: is the acid (lost a proton) H 2 O: is the base (accepted a proton) Now look at the reverse reaction: CH 3 COO- : is the base (accepted a proton) H 3 O+ : is the acid (lost a proton)

Pairs that are related through the loss or gain of a proton are Conjugate pairs Acid 1 Base 2 CH 3 COOH (aq) + H 2 O (aq) Acid 2 Base 1 H 3 O+ (aq) + CH 3 COO - (aq) H 2 O is the conjugate base of H 3 O+ is the conjugate acid of H 2 O

Water can also act as an acid Acid 1 Base 2 H 2 O (aq) + NH 3 (aq) Acid 2 Base 1 NH 4+ (aq) + OH - (aq) H 2 O is the conjugate acid of OHOH- is the conjugate base of H 2 O

The Lewis definition Acid: a substance that can form a covalent bond by accepting an electron pair from a base Base: a substance that have an unshared electron pair with which it can form a covalent bond with an atom, molecule or ion. BF 3 Accepts e’s Acid + NH 3 Donates e’s Base B N

Electrolytes Strong electrolytes are completely ionized in water solution Na. OH is a strong electrolyte Na. OH Na+ + OH- 0. 2 M solution of Na. OH contains: 0. 2 M of Na+ ions and 0. 2 M of OH- ions Weak electrolytes are incompletely ionized in water solution Dissolved molecules exist in equilibrium with their ions in weak electrolytes solutions CH 3 COOH H+ + CH 3 COO -

Water dissociation Pure water is a very weak electrolyte H 2 O + H 2 O H 3 O+ + OH - In simplified form H 2 O H+ + OH - Ionization constant In dilute solutions the concentration of water is constant

Water dissociation constant, at 25°C, Kw = 1. 0× 10 -14

In pure water In acidic solutions In basic solutions
![Example What are [H+] and [OH-] in a 0. 020 M solution of HCl? Example What are [H+] and [OH-] in a 0. 020 M solution of HCl?](http://slidetodoc.com/presentation_image_h/841c4f76f066a5159f2bf4c3dde80b4b/image-10.jpg)
Example What are [H+] and [OH-] in a 0. 020 M solution of HCl? HCl is a strong electrolyte HCl [H+] = 0. 020 M H+ + Cl -

p. H The p. H is the negative logarithm of the hydrogen ion concentration For pure water p. OH of a solution is defined in the same terms

The relationship between p. H and p. OH can be derived from the water dissociation constant Take the logarithm of each term Multiply by -1

The p. H scale 0 1 2 4 Increasing acidity 7 9 11 Increasing alkalinity 14

Example For a 0. 05 M HCl solution a. Calculate b. Calculate p. H c. Calculate p. OH a. [H+] = 0. 05 M &

b. c.
![Example If p. H for a solution =10. 60 Determine [H+] Example If p. H for a solution =10. 60 Determine [H+]](http://slidetodoc.com/presentation_image_h/841c4f76f066a5159f2bf4c3dde80b4b/image-16.jpg)
Example If p. H for a solution =10. 60 Determine [H+]

Weak electrolytes Acetic acid as an example CH 3 COOH H+ + CH 3 COO - Ca 0 0 Ca - x x x Assume x: too small

For a weak base BOH B+ + OH -

Example What is the p. H for a 0. 080 M solution of acetic acid? (Ka=1. 80× 10 -5)

Example What is the p. H for a 0. 200 M solution of NH 3? (Kb=1. 80× 10 -5)

Ions that function as acids and bases a. Anions derived from weak acids CH 3 COONa KNO 2 Na. CN form basic solutions. b. Cations derived from weak bases NH 4 NO 3 Fe. Br 2 form acidic solutions. Al. Cl 3

Anions derived from weak acids CH 3 COO - +H 2 O CH 3 COOH + OH………………. . ………… 1 ………. …… 2 ………………. … 3 Water dissociation ……… 4


Generalize the equation For anions derived from weak acids

Cations derived from weak bases Examples NH 4 NO 3 Fe. Br 2 Al. Cl 3 General equation for cations derived from weak bases:

Example What is the p. H of a 0. 050 M solution of sodium acetate CH 3 COONa? Given that Kacetic acid = 1. 80× 10 -5

Example What is the p. H of a 0. 10 M ammonium chloride NH 4 Cl? Given that Kbase = 1. 80× 10 -5

Buffer solutions Are solutions capable of maintaining their p. H at some fairly constant value even when small amounts of acids or base are added A buffer solution can be prepared from Both a weak acid and a salt of the acid Or Both a weak base and a salt of the base Examples of buffer solutions Acetic acid + sodium acetate CH 3 COOH + CH 3 COO - Ammonia +ammonium chloride NH 3 + NH 4 Cl

Calculating the p. H of buffer solutions weak acid + salt of the acid weak base + salt of the base Henderson-Hasselbalch equations In general, the ratio of ionic species to molecular species for an effective buffer should be between 1/10 and 10/1. Applying the equation to get the p. H range : A buffer solution can be prepared with a p. H of any value between (p. Ka+1) and (p. Ka - 1)

Example What is the p. H of a solution made by adding 2. 05 g of Sodium acetate (CH 3 COONa) into one liter of 0. 09 M acetic acid (CH 3 COOH)? Kacetic acid = 1. 80× 10 -5 M. wt. of CH 3 COONa = 82. 0 g/mol

Example What weight of CH 3 COONa should be added to 1. 0 L of 0. 1 M CH 3 COOH to prepare a buffer solution with a p. H of 5. 0

Example 2. 45× 10 -3 g of Na. CN is added to 500 m. L of 0. 1 M HCN. The p. H of the solution =6. 4 Determine KHCN

Solubility product If an “insoluble” or “slightly soluble” salt is placed in water, An equilibrium is established Ag. Cl (s) Ag+ (aq) + Cl- (aq) The equilibrium constant is Since the concentration of a pure solid is a constant, we can write KSP is called a solubility product

Mg(OH)2 (s) Bi 2 S 3 (s) Mg 2+ (aq) + 2 OH- (aq) 2 Bi 3+ (aq) + 3 S 2 - (aq) The solubility of a salt usually varies widely with temperature, the numerical value of KSP for a salt changes with temperature.

Example At 25ºC, 0. 00188 g of Ag. Cl dissolves in 1 L of water. What is the solubility product of Ag. Cl? Ag. Cl (s) Ag+ (aq) + Cl- (aq)

Example At 25ºC, 7. 8× 10 -5 mol of Ag 2 Cr. O 4 dissolves in 1 L of water. What is the solubility product of Ag 2 Cr. O 4? Ag 2 Cr. O 4 (s) 2 Ag+ (aq) + Cr. O 42 - (aq)

Molar solubility: number of moles dissolved per liter At 25ºC, 0. 00188 g of Ag. Cl dissolves in 1 L of water. Ag. Cl (s) Ag+ (aq) + Cl- (aq) S S

Ca. CO 3 (s) Ca 2+ (aq) + CO 32 - (aq) S S

Example What is the relation between solubility, S, and KSP for Ca 3(PO 4)2? Ca 3(PO 4)2 (s) 3 Ca 2+ (aq) + 2 PO 43 - (aq) 3 S 2 S

Example What is the solubility, S, of Ba. F 2? Ba. F 2 (s) Ba 2+ (aq) + 2 F- (aq)

Example What is the relation between solubility, S, and KSP for Ag 3 PO 4? Ag 3 PO 4 (s) 3 Ag+ (aq) + PO 43 - (aq) 3 S S

Example The solubility of Ca. SO 4 is 0. 67 g/L at certain temperature. What is KSP for Ca. SO 4? Determine S in units of M Ca. SO 4 (s) Ca 2+ (aq) + SO 42 -- (aq)

Example What is p. H and p. OH for a solution of Fe(OH)2? given that KSP= 1. 6× 10 -14. Fe(OH)2 (s) Fe 2+ (aq) + 2 OH- (aq) S 2 S

- Slides: 44