Acids and Bases Science 10 Examples of Acids

Acids and Bases Science 10
Examples of Acids • • • Lemons Stomach acid Acid rain Battery acid Vinegar

Characteristics of Acids • • • Sour taste Can burn the skin Conducts electricity Neutralizes a base Can dissolve in water
Examples of Bases • • • Bleach Baking soda Soap Windex Tonic water

Characteristics of Bases • • • Bitter taste Slippery texture Can burn skin Conducts electricity Neutralizes acids Can dissolve in water

Acids, Bases, Ions and Indicators • In 1884, Svante Arrhenius, a Swedish chemist defined an acid and base.
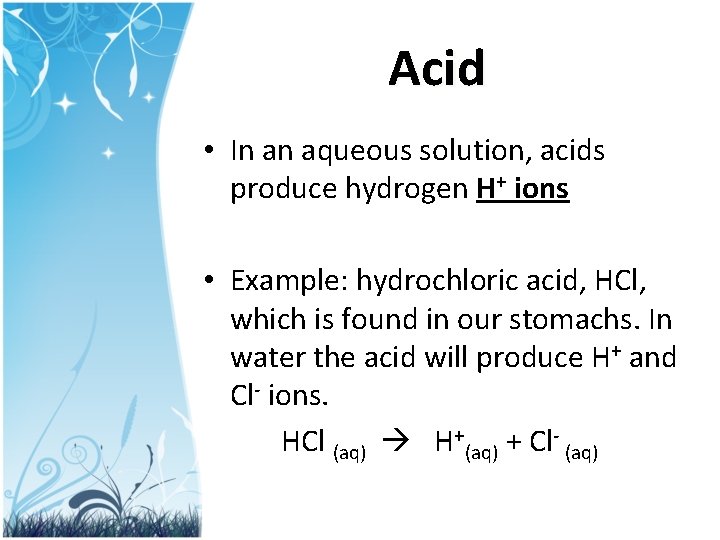
Acid • In an aqueous solution, acids produce hydrogen H+ ions • Example: hydrochloric acid, HCl, which is found in our stomachs. In water the acid will produce H+ and Cl- ions. HCl (aq) H+(aq) + Cl- (aq)
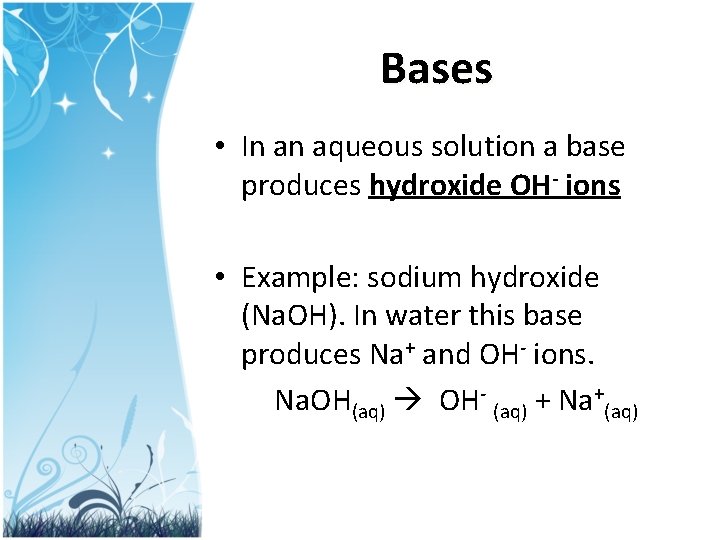
Bases • In an aqueous solution a base produces hydroxide OH- ions • Example: sodium hydroxide (Na. OH). In water this base produces Na+ and OH- ions. Na. OH(aq) OH- (aq) + Na+(aq)
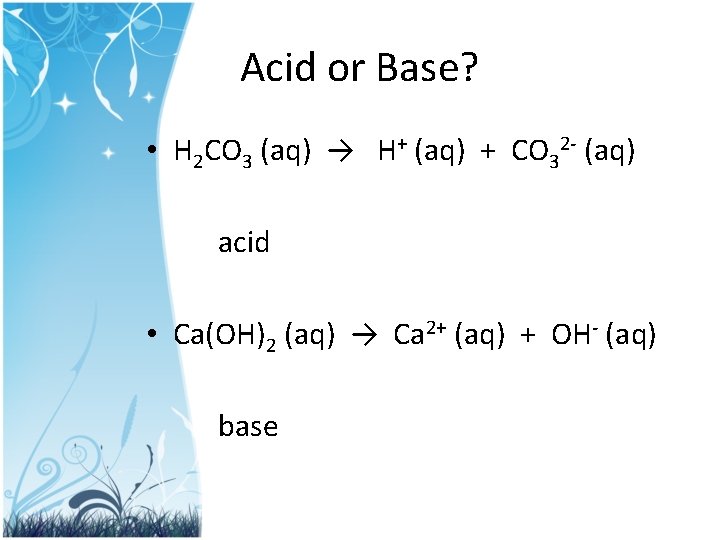
Acid or Base? • H 2 CO 3 (aq) → H+ (aq) + CO 32 - (aq) acid • Ca(OH)2 (aq) → Ca 2+ (aq) + OH- (aq) base
• Most acids and bases are clear and colorless. You need an indicator to tell them apart. • Indicator: a chemical that changes color as the concentration of H+ or OH- changes. • There are many different indicators such as Litmus, phenolphthalein and even cabbage juice!

Litmus Test • Red and blue litmus paper – Acids turn blue litmus paper red. – Bases turn red litmus paper blue – Neutral substances will not change the colour of red OR blue litmus paper.
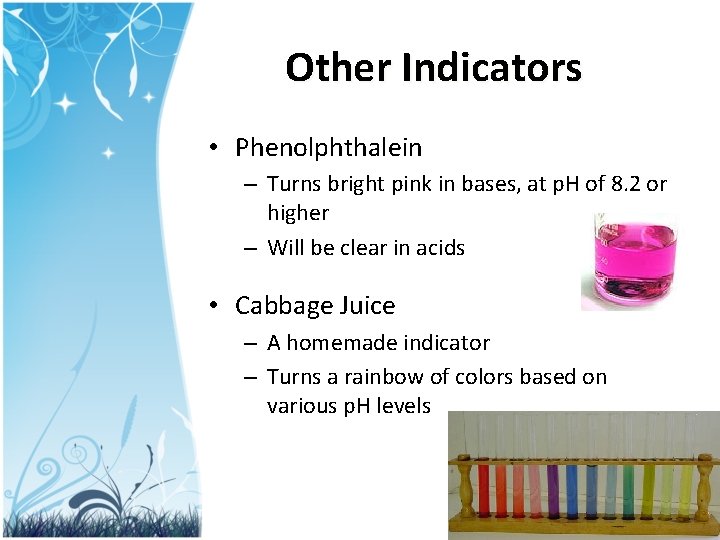
Other Indicators • Phenolphthalein – Turns bright pink in bases, at p. H of 8. 2 or higher – Will be clear in acids • Cabbage Juice – A homemade indicator – Turns a rainbow of colors based on various p. H levels

To do: • Read Pages 156 -158 • Notes – Acids and Bases – Questions: • p. 161, #’s 11, 12, 15 • p. 173, #’s 4, 5
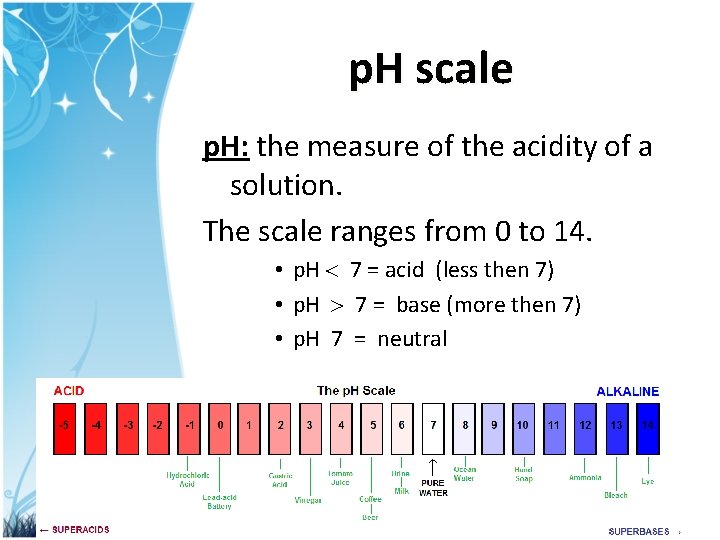
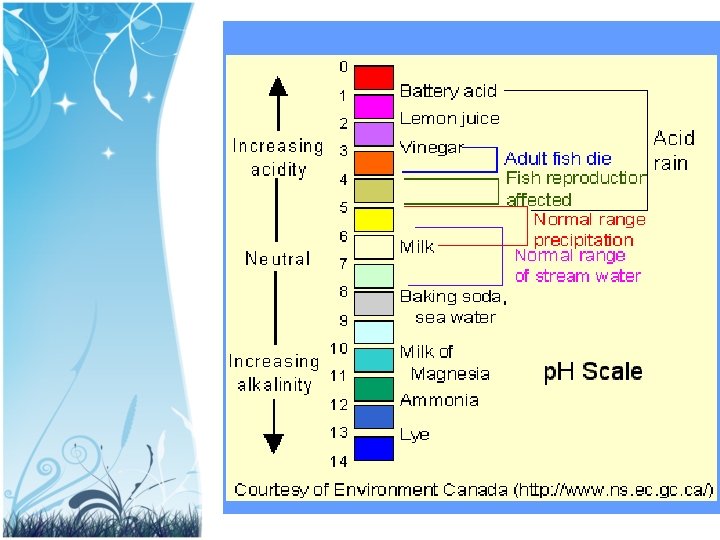
p. H scale p. H: the measure of the acidity of a solution. The scale ranges from 0 to 14. • p. H 7 = acid (less then 7) • p. H 7 = base (more then 7) • p. H 7 = neutral

Acid, Base or Neutral? • H 2 O ↔ H+ (aq) + OH- (aq) –Neutral

• Please note that the aforementioned indicators (litmus and Phenolphthalein do not give an exact p. H, they only tell you if your solution is an acid or a base)

p. H Paper • You compare the colour with a given chart • Instead of telling you whether something is an acid or a base it gives you the exact p. H. Source: http: //escalade. nbed. nb. ca/images/ex 15. jpg

• p. H meter

Acid or Base? • milk (6. 6) – Acid (H+ > OH-) • Baking Soda (8. 7) – Base (H+ < OH-) • Pure water (7. 0) – neutral (H+ = OH-)

To Do: • Read pages 159 -160 • Notes – p. H scale – Questions: • p. 161, #’s 13, 16 • p. 173 #’s 9, 11

Neutralization Reactions • An acid and a base react together to form a new compound (a salt) and water. It’s a double replacement reaction! • The general equation is: Acid + Base → Salt + Water • Example: HCl + Na. OH → Na. Cl + H 2 O

• So the p. H of the new products is close to 7 (neutral). – Examples of neutralization • Antacids • Calcium carbonate is added to acidic lakes.

Practice • Video – Acids and Bases • Notes • Practice Sheet
- Slides: 24