Acids and Bases Properties Reactions and Calculating p

Acids and Bases Properties, Reactions, and Calculating p. H

Standards • • • **STANDARD SET 5: Acids and Bases 5. Acids, bases, and salts are three classes of compounds that form ions in water solutions. As a basis for understanding this concept: a. Students know the observable properties of acids, bases, and salt solutions. • 5. b. Students know acids are hydrogen-ion-donating and bases are hydrogen-ionaccepting substances. • 5. c. Students know strong acids and bases fully dissociate and weak acids and bases partially dissociate. • 5. d. Students know how to use the p. H scale to characterize acid and base solutions. • 5. e. * Students know the Arrhenius, Brønsted-Lowry, and Lewis acid–base definitions. • 5. f. * Students know how to calculate p. H from the hydrogen-ion concentration. • 5. g. * Students know buffers stabilize p. H in acid–base reactions.
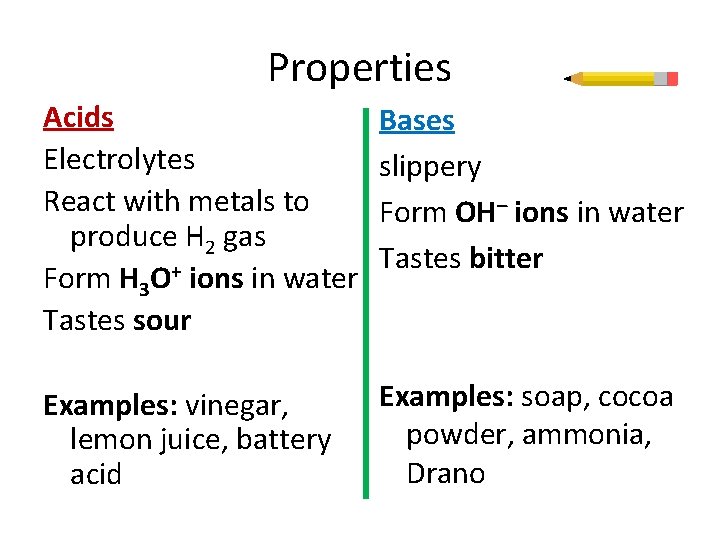
Properties Acids Electrolytes React with metals to produce H 2 gas Form H 3 O+ ions in water Tastes sour Bases slippery Form OH– ions in water Tastes bitter Examples: vinegar, lemon juice, battery acid Examples: soap, cocoa powder, ammonia, Drano
![Calculating p. H [H 3 H+ O +] = 10 –p. H concentration= 1 Calculating p. H [H 3 H+ O +] = 10 –p. H concentration= 1](http://slidetodoc.com/presentation_image_h2/3379e8c9b9637efdb5debf6b66339dc4/image-6.jpg)
Calculating p. H [H 3 H+ O +] = 10 –p. H concentration= 1 × 10 p. H = 12 – 12
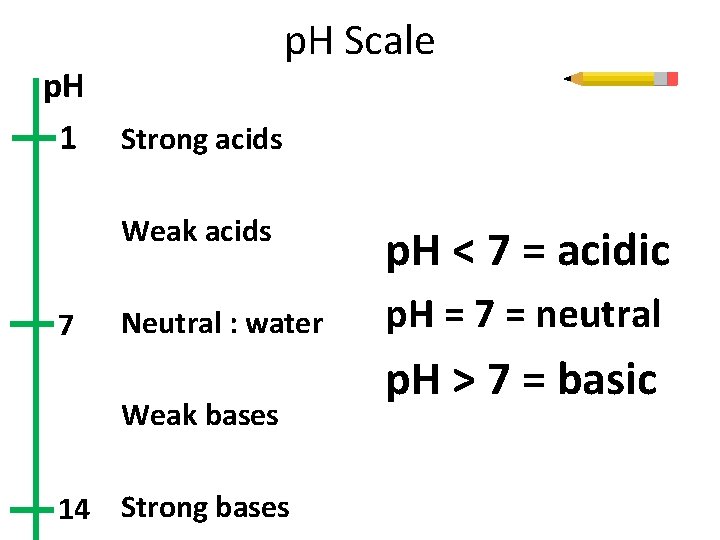
p. H 1 7 p. H Scale Strong acids Weak acids p. H < 7 = acidic Neutral : water p. H = 7 = neutral Weak bases 14 Strong bases p. H > 7 = basic
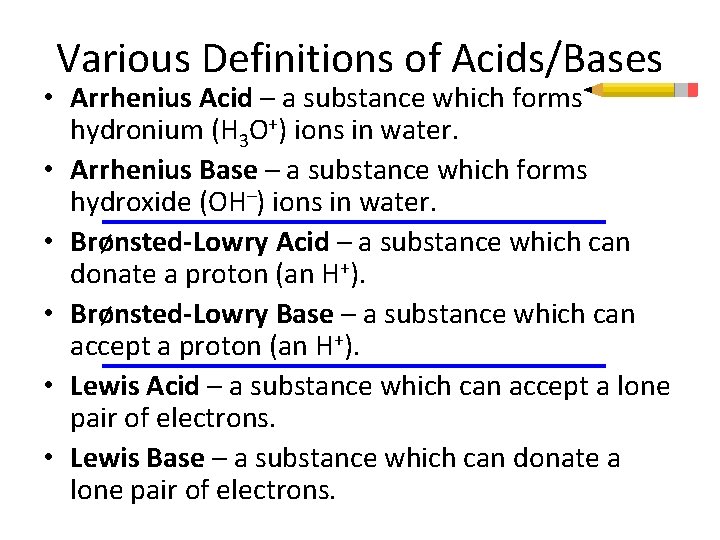
Various Definitions of Acids/Bases • Arrhenius Acid – a substance which forms hydronium (H 3 O+) ions in water. • Arrhenius Base – a substance which forms hydroxide (OH–) ions in water. • Brønsted-Lowry Acid – a substance which can donate a proton (an H+). • Brønsted-Lowry Base – a substance which can accept a proton (an H+). • Lewis Acid – a substance which can accept a lone pair of electrons. • Lewis Base – a substance which can donate a lone pair of electrons.
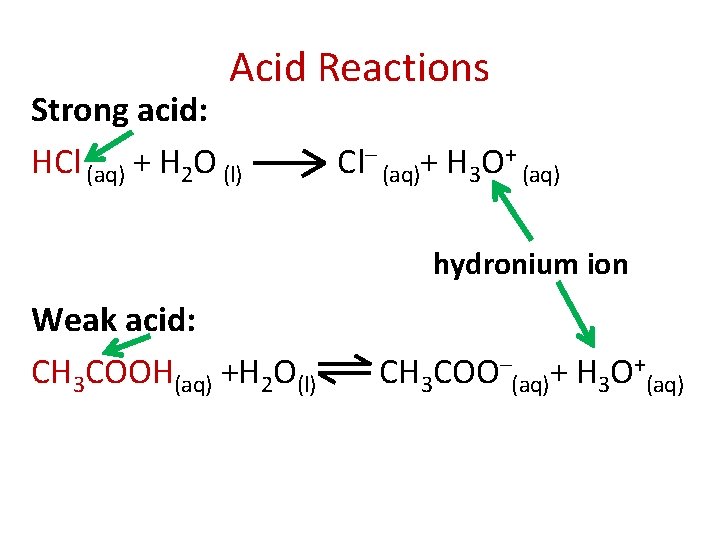
Acid Reactions Strong acid: HCl (aq) + H 2 O (l) Cl– (aq)+ H 3 O+ (aq) hydronium ion Weak acid: CH 3 COOH(aq) +H 2 O(l) CH 3 COO–(aq)+ H 3 O+(aq)
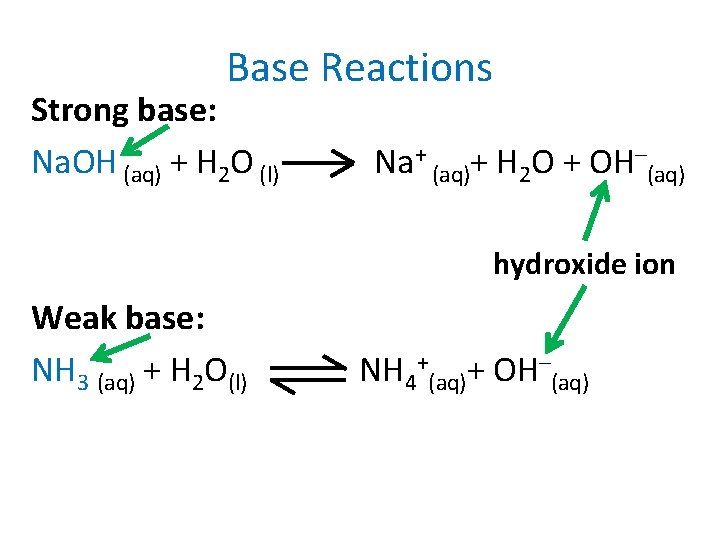
Base Reactions Strong base: Na. OH (aq) + H 2 O (l) Na+ (aq)+ H 2 O + OH–(aq) hydroxide ion Weak base: NH 3 (aq) + H 2 O(l) NH 4+(aq)+ OH–(aq)
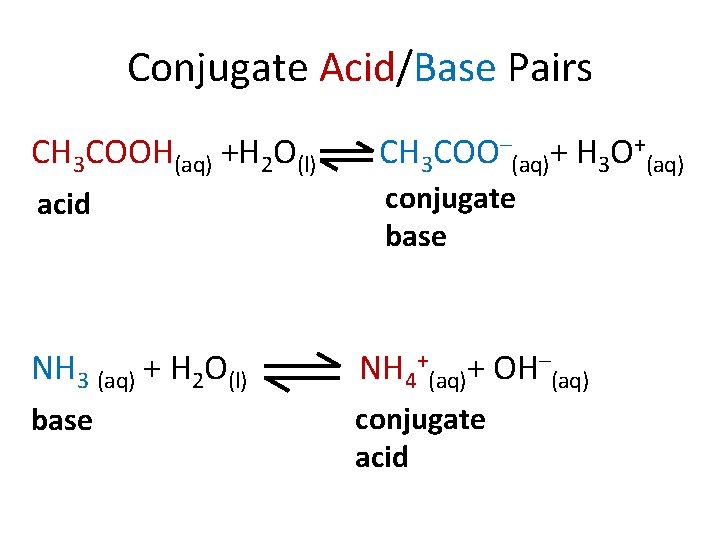
Conjugate Acid/Base Pairs CH 3 COOH(aq) +H 2 O(l) acid CH 3 COO–(aq)+ H 3 O+(aq) conjugate base NH 3 (aq) + H 2 O(l) NH 4+(aq)+ OH–(aq) base conjugate acid
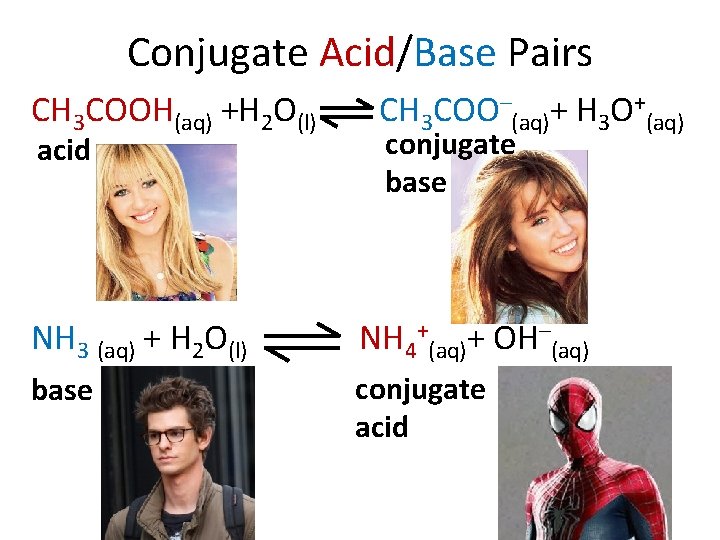
Conjugate Acid/Base Pairs CH 3 COOH(aq) +H 2 O(l) acid CH 3 COO–(aq)+ H 3 O+(aq) conjugate base NH 3 (aq) + H 2 O(l) NH 4+(aq)+ OH–(aq) base conjugate acid
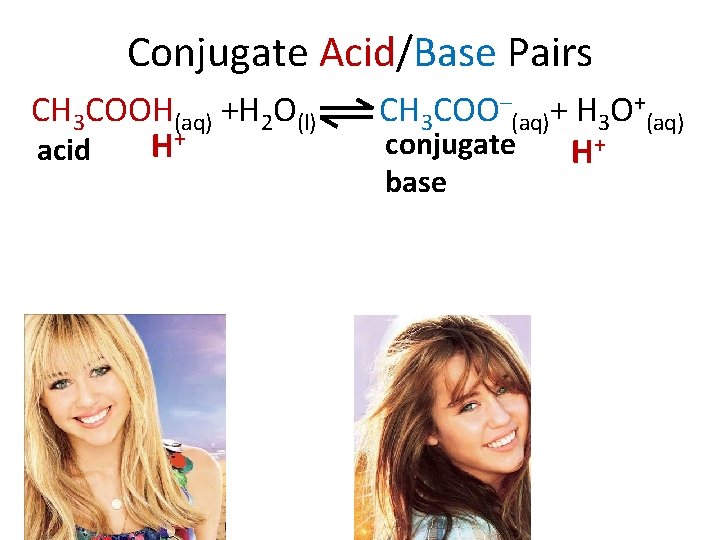
Conjugate Acid/Base Pairs CH 3 COOH(aq) +H 2 O(l) H+ acid CH 3 COO–(aq)+ H 3 O+(aq) conjugate H+ base
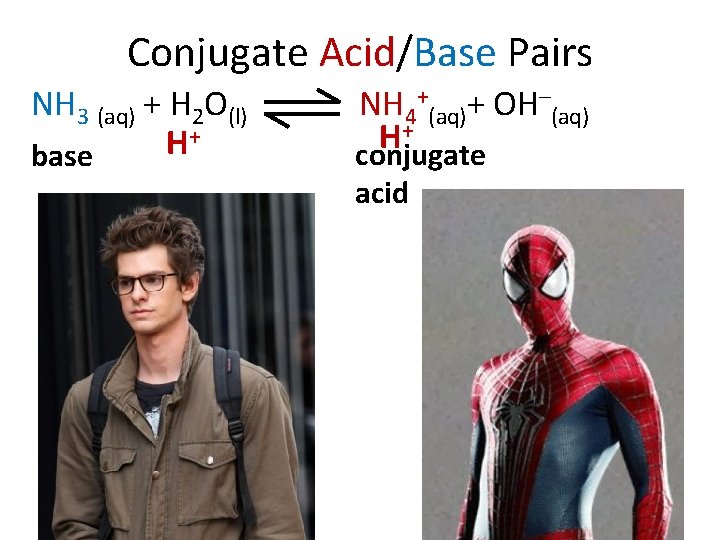
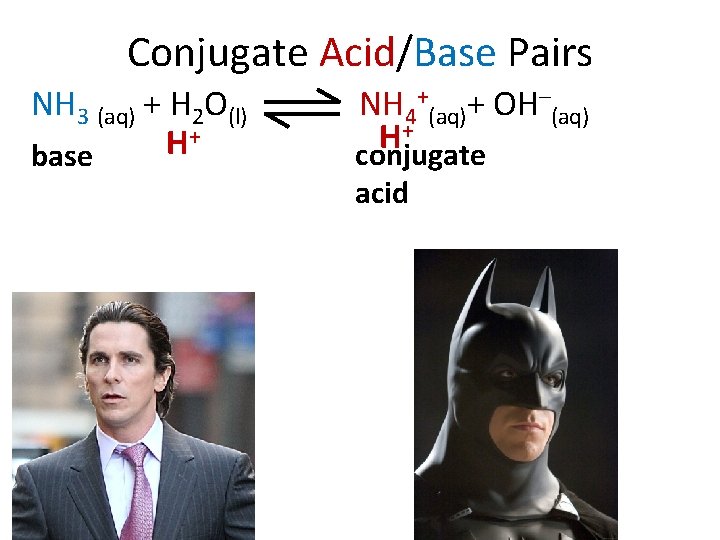
Conjugate Acid/Base Pairs NH 3 (aq) + H 2 O(l) + H base NH 4+(aq)+ OH–(aq) + H conjugate acid
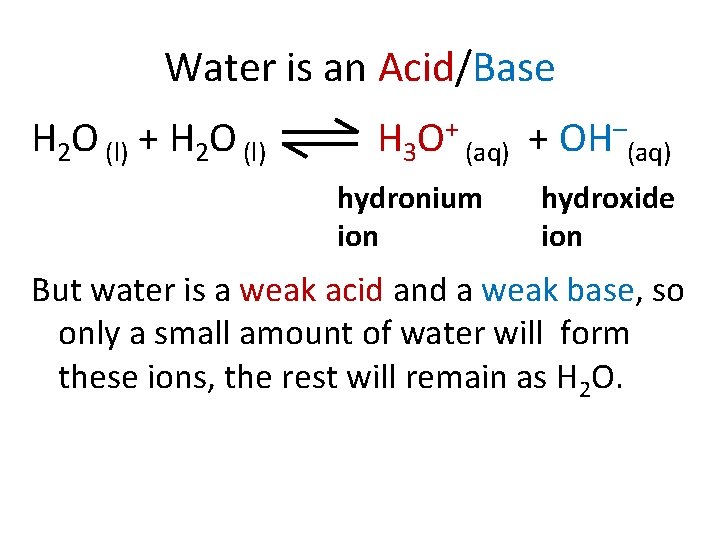
Water is an Acid/Base H 2 O (l) + H 2 O (l) H 3 O+ (aq) + OH–(aq) hydronium ion hydroxide ion But water is a weak acid and a weak base, so only a small amount of water will form these ions, the rest will remain as H 2 O.

Measuring the Strength of Acids/Bases We often speak of acid concentrations in molarity (ex. 2. 0 M HCl solution, aka 2. 0 mol HCl 1 L solution ) But which is stronger 2. 0 M HCl solution or 2. 0 M CH 3 COOH solution? It’s more important to know the concentration of hydronium ions. [H+] really means [H 3 O+]
![Calculating p. H = – log 10[H 3 O+] Example: If [H 3 O+] Calculating p. H = – log 10[H 3 O+] Example: If [H 3 O+]](http://slidetodoc.com/presentation_image_h2/3379e8c9b9637efdb5debf6b66339dc4/image-17.jpg)
Calculating p. H = – log 10[H 3 O+] Example: If [H 3 O+] = 1 x 10– 5 M, what is the p. H? p. H = – log 10[1 x 10– 5] p. H = 5 Back-calculating concentration (– 1) x – log 10[H 3 O+] = p. H x (– 1) log 10[H 3 O+] = –p. H 10 [H 3 10 + O] = 10 –p. H
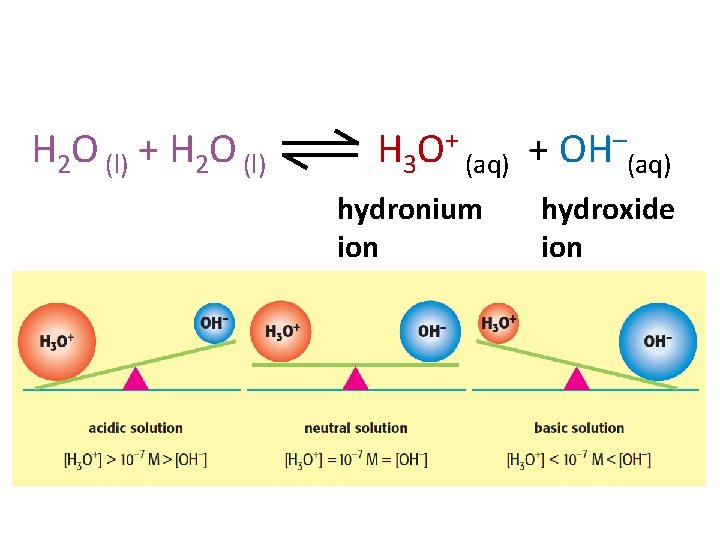
H 2 O (l) + H 2 O (l) H 3 O+ (aq) + OH–(aq) hydronium ion hydroxide ion
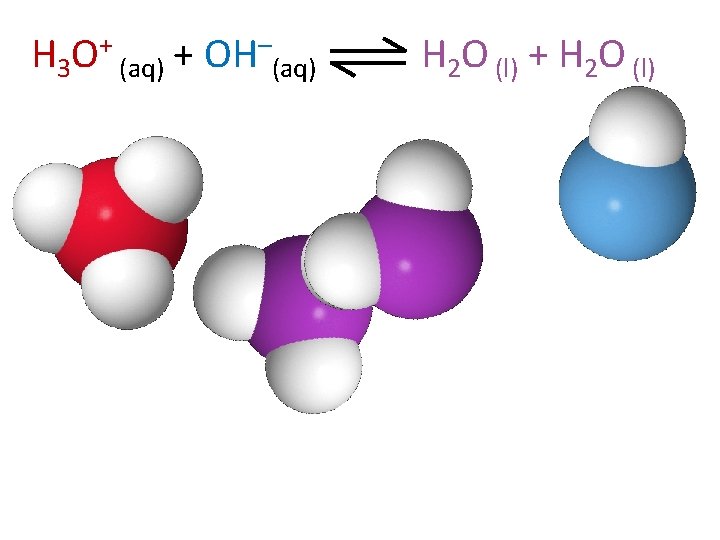
H 3 O+ (aq) + OH–(aq) H 2 O (l) + H 2 O (l)
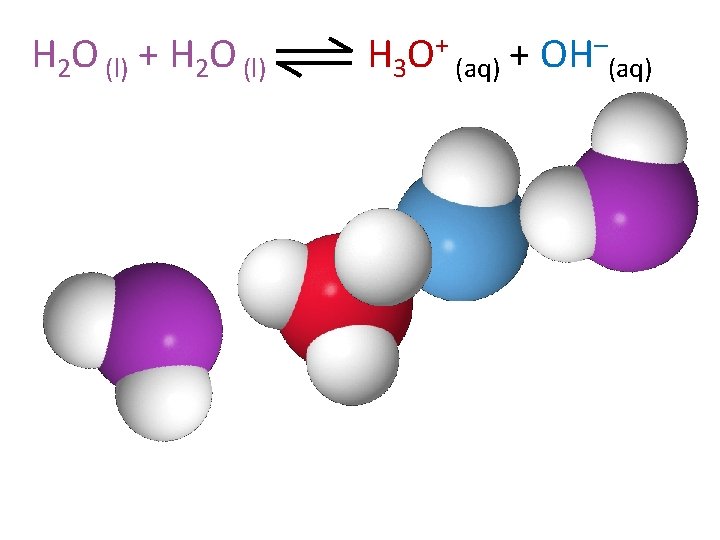
H 2 O (l) + H 2 O (l) H 3 O+ (aq) + OH–(aq)
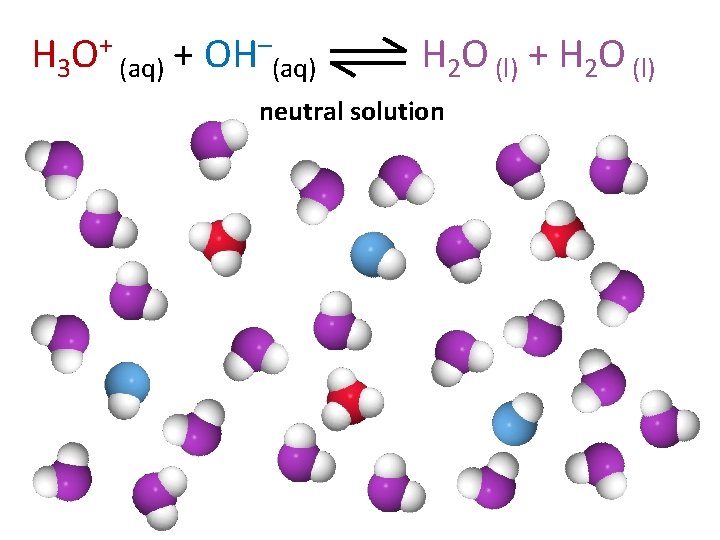
H 3 O+ (aq) + OH–(aq) H 2 O (l) + H 2 O (l) neutral solution
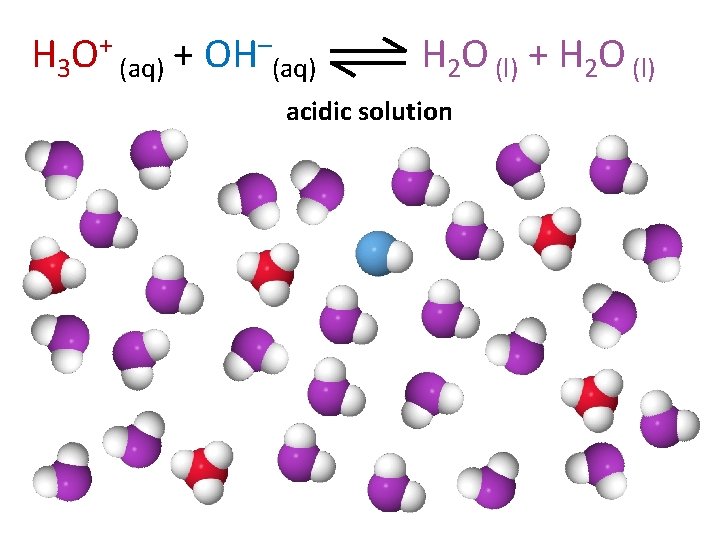
H 3 O+ (aq) + OH–(aq) H 2 O (l) + H 2 O (l) acidic solution
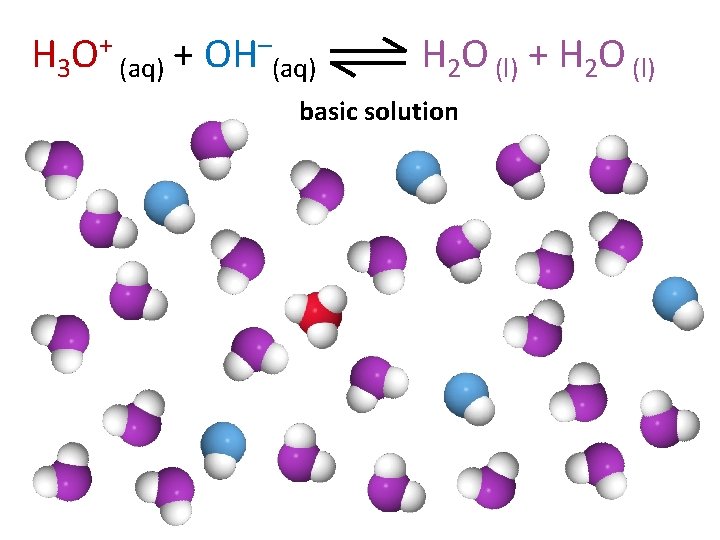
H 3 O+ (aq) + OH–(aq) H 2 O (l) + H 2 O (l) basic solution
![Calculating [OH–] from [H 3 O+] H 2 O (l) + H 2 O Calculating [OH–] from [H 3 O+] H 2 O (l) + H 2 O](http://slidetodoc.com/presentation_image_h2/3379e8c9b9637efdb5debf6b66339dc4/image-24.jpg)
Calculating [OH–] from [H 3 O+] H 2 O (l) + H 2 O (l) H 3 O+ (aq) + OH–(aq) Kw = [H 3 O+]·[OH–] Kw = 1 x 10– 14 always this number Example: If [H 3 O+]= 1 x 10– 5 M, what is [OH–]? 1 x 10– 14 = [1 x 10– 5 M]·[OH–] [1 x 10– 5 M] 1 x 10– 9 M = [OH–]
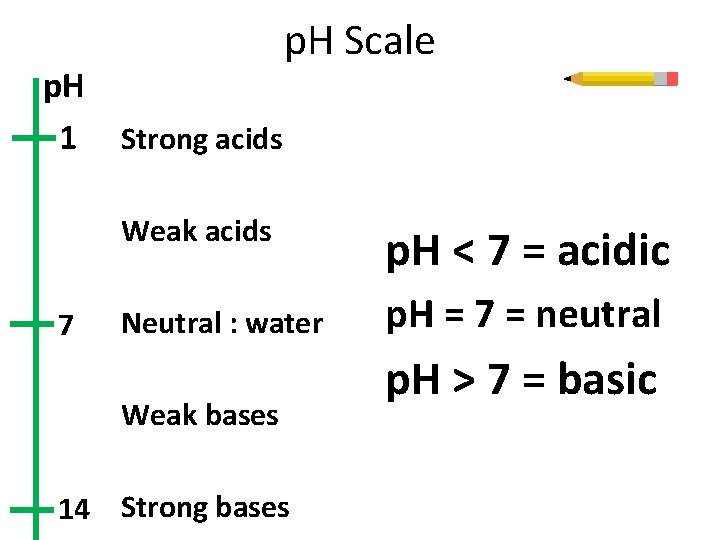
p. H 1 7 p. H Scale Strong acids Weak acids p. H < 7 = acidic Neutral : water p. H = 7 = neutral Weak bases 14 Strong bases p. H > 7 = basic
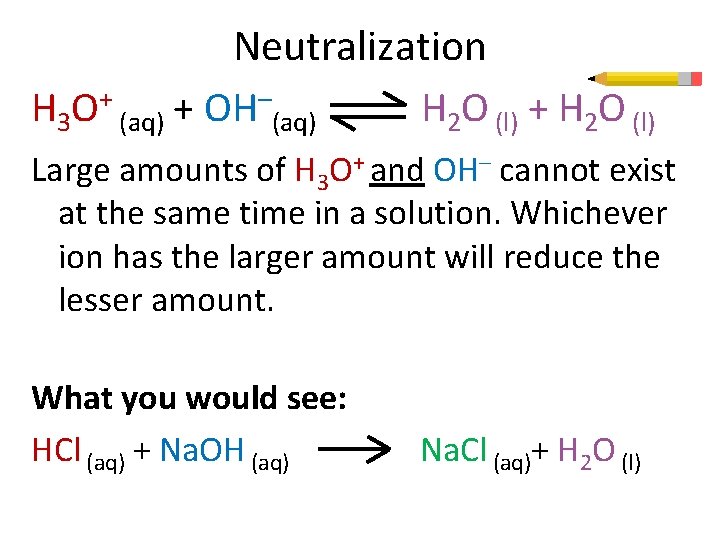
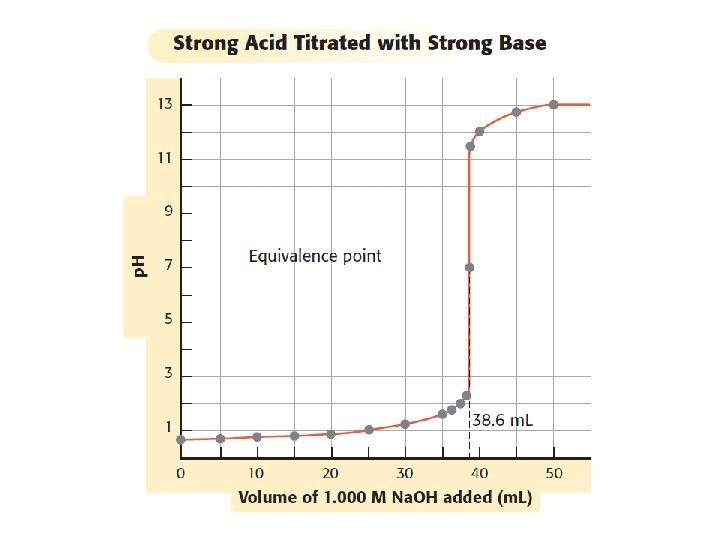
Neutralization H 3 O+ (aq) + OH–(aq) H 2 O (l) + H 2 O (l) Large amounts of H 3 O+ and OH– cannot exist at the same time in a solution. Whichever ion has the larger amount will reduce the lesser amount. What you would see: HCl (aq) + Na. OH (aq) Na. Cl (aq)+ H 2 O (l)
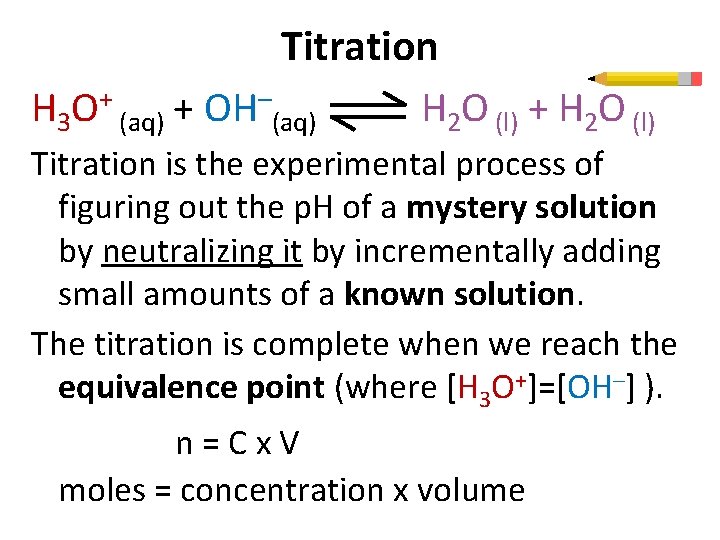
Titration H 3 O+ (aq) + OH–(aq) H 2 O (l) + H 2 O (l) Titration is the experimental process of figuring out the p. H of a mystery solution by neutralizing it by incrementally adding small amounts of a known solution. The titration is complete when we reach the equivalence point (where [H 3 O+]=[OH–] ). n=Cx. V moles = concentration x volume
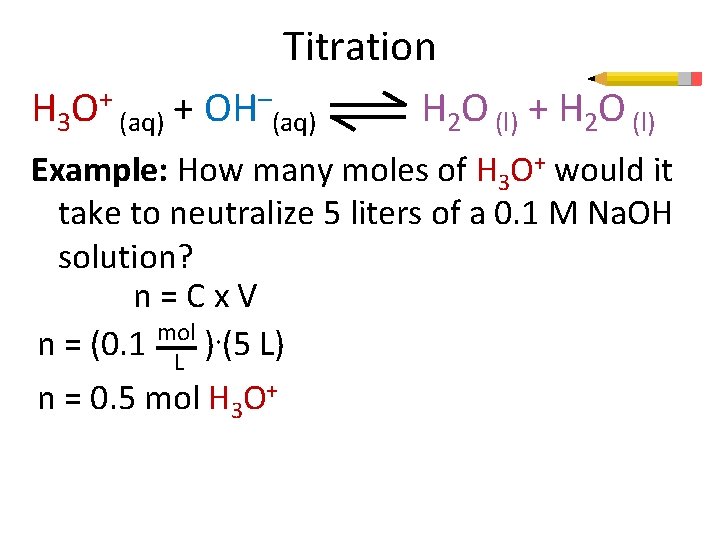
Titration H 3 O+ (aq) + OH–(aq) H 2 O (l) + H 2 O (l) Example: How many moles of H 3 O+ would it take to neutralize 5 liters of a 0. 1 M Na. OH solution? n=Cx. V n = (0. 1 mol ) ·(5 L) L n = 0. 5 mol H 3 O+
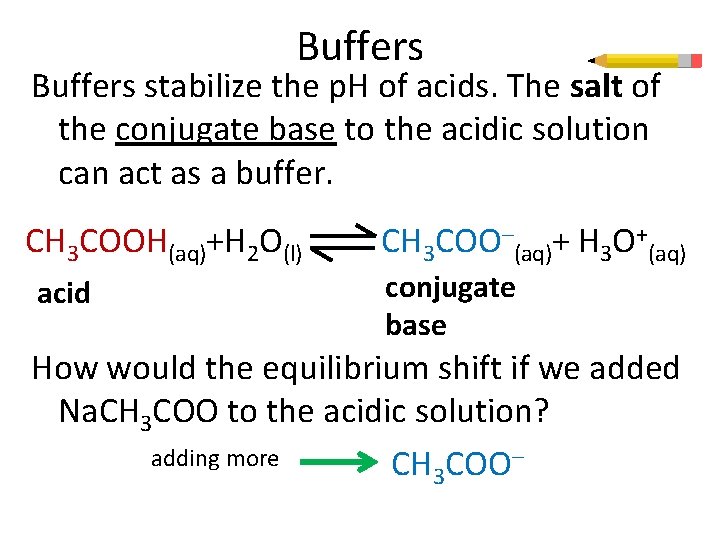
Buffers stabilize the p. H of acids. The salt of the conjugate base to the acidic solution can act as a buffer. CH 3 COOH(aq)+H 2 O(l) acid CH 3 COO–(aq)+ H 3 O+(aq) conjugate base How would the equilibrium shift if we added Na. CH 3 COO to the acidic solution? adding more CH 3 COO–
H He Li Be B C N O F Na Mg Al Si P S Cl Ar K Ca Ne Br Kr I Xe
H He Li Be B C N O F Na Mg Al Si P S Cl Ar K Ca Ne Br Kr I Xe
4 e– in valence shell

Measuring the Strength of Acids/Bases We often speak of acid concentrations in molarity (ex. 2. 0 M HCl solution, aka 2. 0 mol HCl 1 L solution ) But which is stronger 2. 0 M HCl solution or 2. 0 M CH 3 COOH solution? Since we use both strong and weak acids, a more consistent measurement would tell us just the concentration of hydronium ions. [H+] really means [H 3 O+]
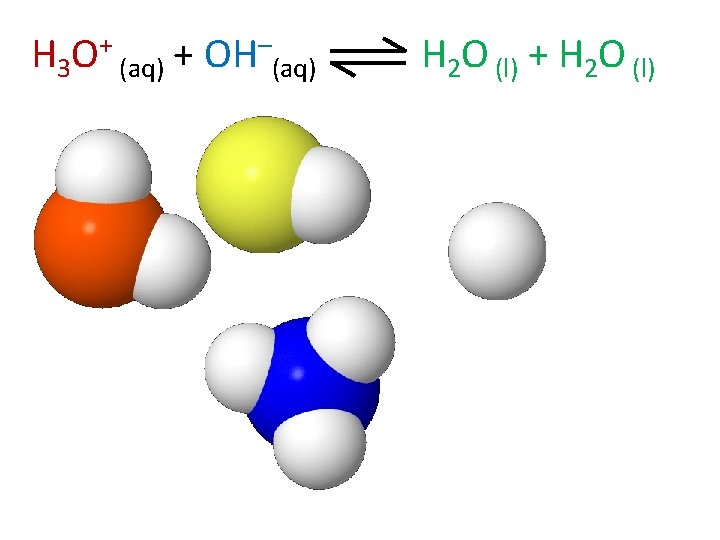
H 3 O+ (aq) + OH–(aq) H 2 O (l) + H 2 O (l)
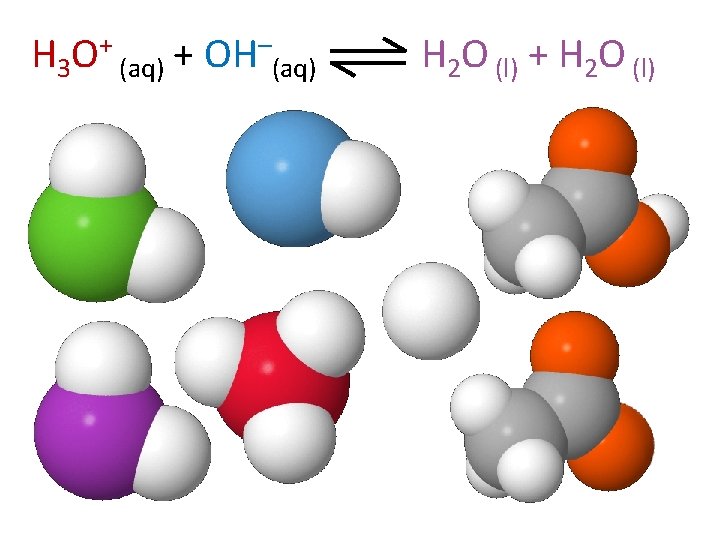
H 3 O+ (aq) + OH–(aq) H 2 O (l) + H 2 O (l)




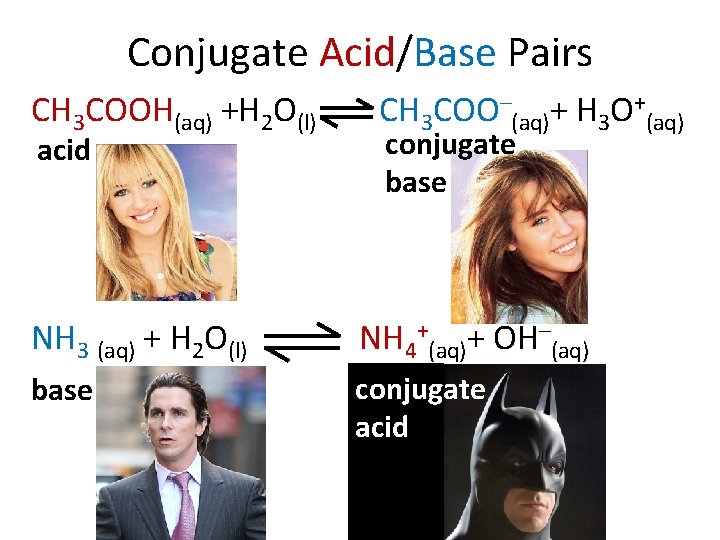
Conjugate Acid/Base Pairs CH 3 COOH(aq) +H 2 O(l) acid CH 3 COO–(aq)+ H 3 O+(aq) conjugate base NH 3 (aq) + H 2 O(l) NH 4+(aq)+ OH–(aq) base conjugate acid
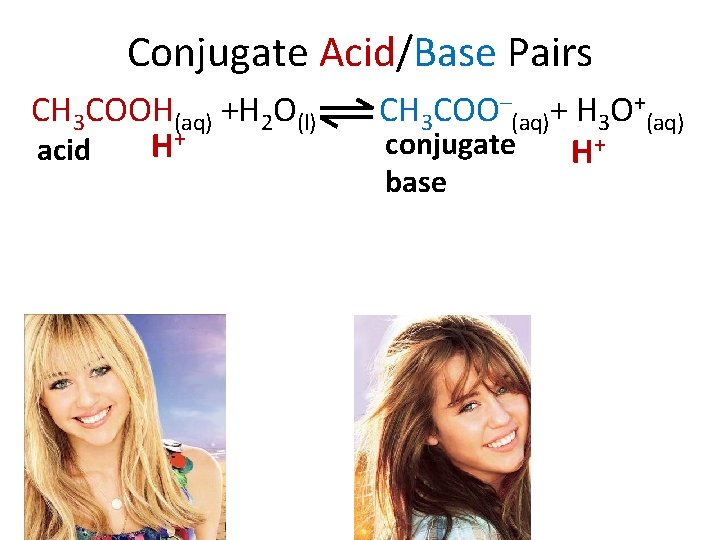
Conjugate Acid/Base Pairs CH 3 COOH(aq) +H 2 O(l) H+ acid CH 3 COO–(aq)+ H 3 O+(aq) conjugate H+ base
Conjugate Acid/Base Pairs NH 3 (aq) + H 2 O(l) + H base NH 4+(aq)+ OH–(aq) + H conjugate acid
- Slides: 45