Acids and Bases Properties of Acids Have a

Acids and Bases

Properties of Acids • • Have a sour taste Changes colors of substances called indicators Aqueous Solutions are electrolytes (conduct electricity) React with metals: Zn + 2 HCl Zn. Cl 2 + H 2 Reacts with carbonates: 2 HCl + Na 2 CO 3 2 Na. Cl + H 2 O + CO 2 Have at least one hydrogen atom attached to a negative ion. Reacts with (-OH) to form water p. H below 7

Naming Acids • Hydrogen comes first you know it’s an acid • The second part determines the name – Ends in “ide”… Acid name begins with hydro, the stem of the anion has the suffix “ic” and then acid is on the end – Ends in “ite”… The stem of the anion has the suffix “ous” and then acid is on the end – Ends in “ate”… The stem of the anion has the suffix “ic” and then acid is on the end – Practice problems 1 -5 pg 579

Properties of Bases • • • Have a bitter taste Changes colors of indicators Feels slippery Reacts with (H+) to form water p. H above 7 No special naming structure just normal rules

Hydrogen Ions and Acidity • Hydrogen Ions from Water – • H 2 O + H 2 O H 3 O+ + -OH or H 2 O H+ + -OH – – When water self-ionizes [H+] = [-OH] = 1. 0 x 10 -7 -OH] = (1. 0 x 10 -7) = 1. 0 x 10 Kw = [H+][ -14 – Acid solutions-substance is added to water to increase [H+] so [H+] >1. 0 x 10 -7 Basic solutions-substance is added to water to increase [-OH] so [-OH] >1. 0 x 10 -7 – • Water self-ionizes Practice problems #6 -7 pg 582

The p. H Concept • • • p. H is a measurement of the acidity of solutions. p. H=-log [H+] If p. H>7 the solution is basic. If p. H<7 the solution is acidic. If p. H=7 the solution is neutral. p. OH is a measurement of the basicity of solutions. p. OH=-log [OH-] p. H + p. OH = 14. Also [H+][OH-] = 1. 0 x 10 -14 Practice problems #8, 9 and 12, 13 pg 586 and 588
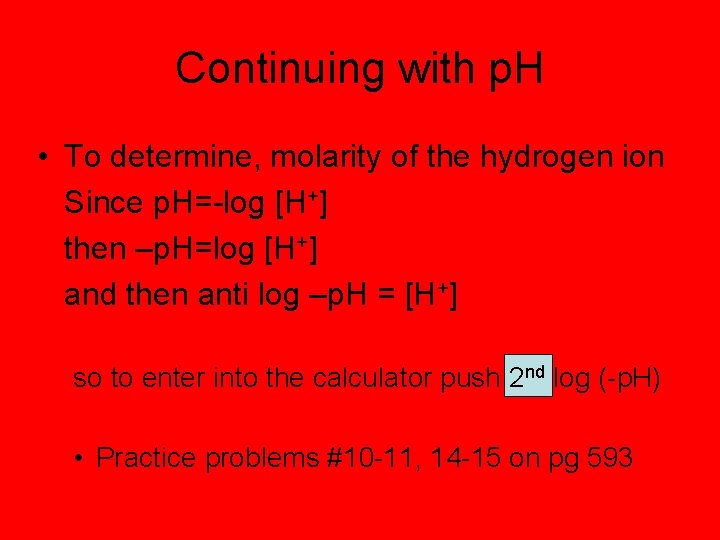
Continuing with p. H • To determine, molarity of the hydrogen ion Since p. H=-log [H+] then –p. H=log [H+] and then anti log –p. H = [H+] so to enter into the calculator push 2 nd log (-p. H) • Practice problems #10 -11, 14 -15 on pg 593

Measuring p. H • Indicators – For approximate, preliminary or for small samples – Chemicals that react with [H+] or [-OH] ions to produce color changes – Often affected by temperature – Solutions that are colored are more difficult to test – Examples… • • Phenothalein Methyl Red Bromothymol Blue Litmus Paper p. H Paper Universal Indicator Cabbage Juice And others…

• p. H meter or probe – For measuring precise and continuous values – Have to be calibrated by immersing electrode in a known p. H solution

Acid Strength • Strong Acids – – – Strong acids dissociate completely in water: HCl, HBr, HI, HNO 3, H 2 SO 4, HCl. O 4 HCl + H 2 O H 3 O+ + Cl-. This reaction does not reach equilibrium because all of the HCl dissociates. What is the p. H of a solution if 0. 10 mol of HCl is dissolved in 250 m. L water? • • • Since HCl is a strong acid, it will all dissociate. The initial [HCl] is 0. 10 mol/0. 25 L = 0. 040 M. Since 1 HCl forms 1 H 3 O+, the [H 3 O+] = 0. 040 M Since [H 3 O+] = 0. 040 M, p. H = -log 0. 040 = 1. 4

• Weak Acids – – Weak acids do not dissociate completely in water. See Appendix D (Table 20. 8 pg 602) for partial list of weak acids. Acetic acid dissociates according to: • CH 3 COOH(aq) + H 2 O(l) H 3 O+(aq) + CH 3 COO-(aq) and will reach equilibrium! – We can write a special equilibrium expression for weak acids: • • – HA(aq) + H 2 O(l) H 3 O+(aq) + A-(aq) Keq = [H 3 O+] [A-] [HA] [H 2 O] In weak acids, [H 2 O] is large and remains constant so Keq X [H 2 O] = a constant we call Ka so Ka = [H 3 O+] [A-] [HA]
![• Calculations for weak acids – What is the [H 3 O+] in • Calculations for weak acids – What is the [H 3 O+] in](http://slidetodoc.com/presentation_image_h2/16a5fbbf1b7c5f1ead5c2bd0f225b754/image-12.jpg)
• Calculations for weak acids – What is the [H 3 O+] in a 0. 20 M solution of acetic acid? CH 3 COOH(aq) + H 2 O(l) H 3 O+(aq) + CH 3 COO-(aq) Ka = [H 3 O+] [CH 3 COO-] [CH 3 COOH] Ka = 1. 8 x 10 -5 [H 3 O+] [CH 3 COO-] [CH 3 COOH] [initial] 0 0 0. 20 [final] x x 0. 20 – x 1. 8 x 10 -5 = (x) 0. 20 – x This can be solved using the quadratic formula OR since so little acetic acid dissociates, 0. 20 -x = 0. 20 1. 8 x 10 -5 = x 2 x = 1. 9 x 10 -3 M 0. 20
![What is the % ionization? % ionization = [H 3 O+] x 100 initial What is the % ionization? % ionization = [H 3 O+] x 100 initial](http://slidetodoc.com/presentation_image_h2/16a5fbbf1b7c5f1ead5c2bd0f225b754/image-13.jpg)
What is the % ionization? % ionization = [H 3 O+] x 100 initial [CH 3 COOH] = 1. 9 x 10 -3 x 100 = 1. 0% 0. 20 What is the p. H? p. H = -log [H 3 O+] = -log 1. 9 x 10 -3 = 2. 7 • What is the [H 3 O+] in a 0. 20 M solution of methanoic acid? What is the % dissociation? What is the p. H? • What is the [H 3 O+] in a 0. 50 M solution of benzoic acid? What is the % dissociation? What is the p. H?
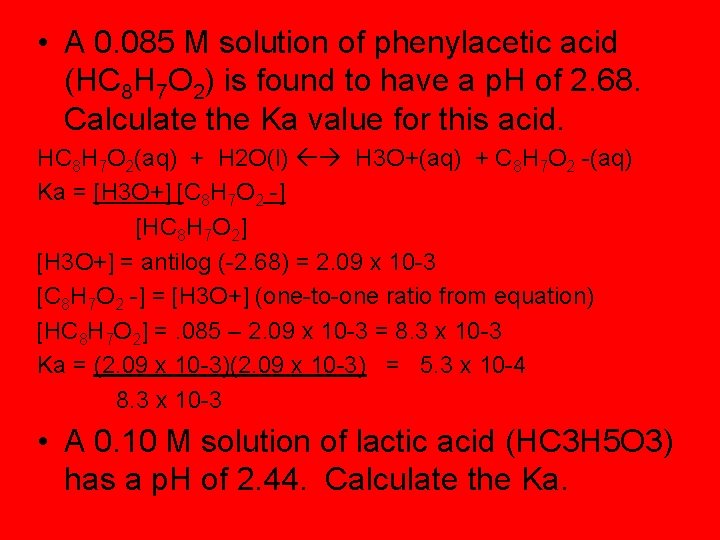
• A 0. 085 M solution of phenylacetic acid (HC 8 H 7 O 2) is found to have a p. H of 2. 68. Calculate the Ka value for this acid. HC 8 H 7 O 2(aq) + H 2 O(l) H 3 O+(aq) + C 8 H 7 O 2 -(aq) Ka = [H 3 O+] [C 8 H 7 O 2 -] [HC 8 H 7 O 2] [H 3 O+] = antilog (-2. 68) = 2. 09 x 10 -3 [C 8 H 7 O 2 -] = [H 3 O+] (one-to-one ratio from equation) [HC 8 H 7 O 2] =. 085 – 2. 09 x 10 -3 = 8. 3 x 10 -3 Ka = (2. 09 x 10 -3) = 5. 3 x 10 -4 8. 3 x 10 -3 • A 0. 10 M solution of lactic acid (HC 3 H 5 O 3) has a p. H of 2. 44. Calculate the Ka.
- Slides: 14