Acids and Bases Presented by Kesler Science Essential

Acids and Bases Presented by Kesler Science

Essential Questions: 1. What are the differences between acids and bases? 2. How are ions related to acids and bases? 3. How do you test for acids and bases using p. H? .

Acids and Bases Acids • A solution that has an excess of H+ ions. • From the Latin word acidus, which means “sharp” or “sour” • Characteristics • Taste sour • React strongly with metals • Dangerous and can burn your skin • Turns blue litmus indicator red • Increase H+ ion concentration in solution © Kesler. Science. com

Acids and Bases • A solution that has an excess of OH- ions. • Another word for base is alkali or alkaline • Characteristics of bases • Bitter taste • Slippery feel • Strong bases are very dangerous and can burn your skin. • Turns red litmus indicator blue • Increase OH- ion concentration in solution © Kesler. Science. com

Quick Action – Acids and Bases Practice Grab a partner and let’s talk about acids and bases. See how many common acids can you list in 30 sec. Think about their characteristics. Go! Now do the same with bases. 30 sec. Go! © Kesler. Science. com

Acids and Bases Acids Uses • • • Acetic acid – vinegar Acetylsalicylic acid – aspirin Ascorbic acid – vitamin C Carbonic acid – soft drinks Citric acid – citrus fruits, artificial flavorings • Hydrochloric acid – stomach acid • Nitric acid – fertilizer, explosives • Sulfuric Acid – car batteries © Kesler. Science. com

Acids and Bases Uses • Ammonium hydroxide – glass cleaner • Aluminum hydroxide – antacids, deodorants • Magnesium hydroxide – laxatives, antacids • Sodium bicarbonate – baking soda • Sodium carbonate – dish detergent • Sodium hydroxide – lye, oven and drain cleaner • Sodium hypochlorite - bleach © Kesler. Science. com
Acids and Bases in Nature • There are many strong acids and bases in nature. • Fruits contain citric acid. • Some are dangerous and used as poisons by insects and animals. • Many plants have acids and bases in their leaves, seeds, or even their sap. © Kesler. Science. com

Acids and Bases in our Bodies • Our stomachs use hydrochloric acid to help digest foods, and also kills bacteria that causes us to get sick. • Our muscles produce lactic acid when we exercise. • Our pancreas uses a base to help with digestion. © Kesler. Science. com

Acids and Bases Svante Arrhenius • Chemist who came up with a way to define acids and bases in 1887 • Believed that acids were substances that produce hydrogen ions [H]+ in solution • Bases were substances that produced hydroxide ions [OH]- in solution. © Kesler. Science. com

Acids and Bases Ions • Form when atoms lose or gain electrons to have full outer valence electron shells. (Octet rule) • Remember electrons have a negative charge. • Cations – lose electrons, + charge • Anions – gain electrons, - charge Sodium loses 1 electron Sodium: Na Oxygen gains 2 electron Oxygen: O © Kesler. Science. com Cation sodium: [Na]+ Anion oxygen: [O]2 -
Quick Action – Acids and Bases Practice 1. Draw a Bohr Model of the elements H, O, Ca, Cl. 2. How many electrons are on the valence shell? 3. Which ones will give away (lose)their valence electrons? 4. Which ones will add (gain) extra electrons? Hint: half or more add extra electrons (gain) half or less give away electrons (lose) © Kesler. Science. com
![Acids and Bases Ions in Acids and Bases • Hydrogen ions [H]+ in water Acids and Bases Ions in Acids and Bases • Hydrogen ions [H]+ in water](http://slidetodoc.com/presentation_image_h2/84a4b05df3534fc18640580ac453c1d9/image-13.jpg)
Acids and Bases Ions in Acids and Bases • Hydrogen ions [H]+ in water mixed with acidic compounds form hydronium ions [H 3 O]+ • Hydrogen ions [H]+ in water mixed with basic compounds form hydroxide ions [OH]- H O O H © Kesler. Science. com H H Hydronium ion [H 3 O]+ Hydroxide ion [OH]-
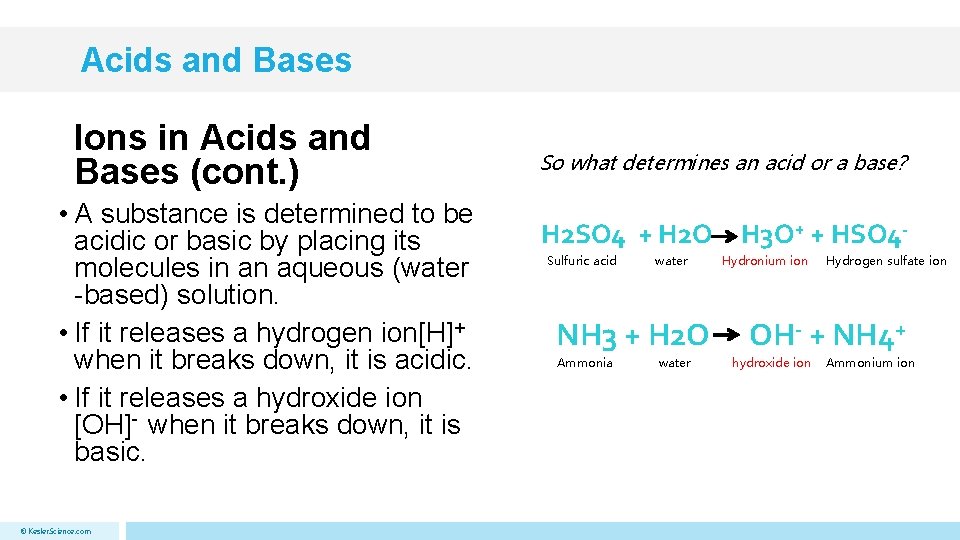
Acids and Bases Ions in Acids and Bases (cont. ) • A substance is determined to be acidic or basic by placing its molecules in an aqueous (water -based) solution. • If it releases a hydrogen ion[H]+ when it breaks down, it is acidic. • If it releases a hydroxide ion [OH]- when it breaks down, it is basic. © Kesler. Science. com So what determines an acid or a base? H 2 SO 4 + H 2 O Sulfuric acid water NH 3 + H 2 O Ammonia water H 3 O+ + HSO 4 Hydronium ion Hydrogen sulfate ion OH- + NH 4+ hydroxide ion Ammonium ion
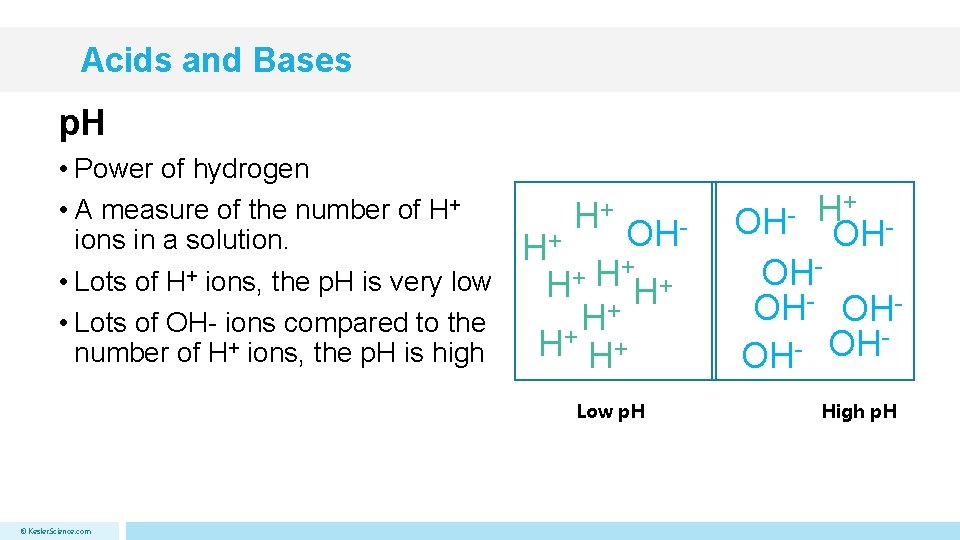
Acids and Bases p. H • Power of hydrogen • A measure of the number of H+ ions in a solution. • Lots of H+ ions, the p. H is very low • Lots of OH- ions compared to the number of H+ ions, the p. H is high H+ OH- H+ + H H+ H+ Low p. H © Kesler. Science. com OH- H+ OH OHOH- OHOH OH High p. H
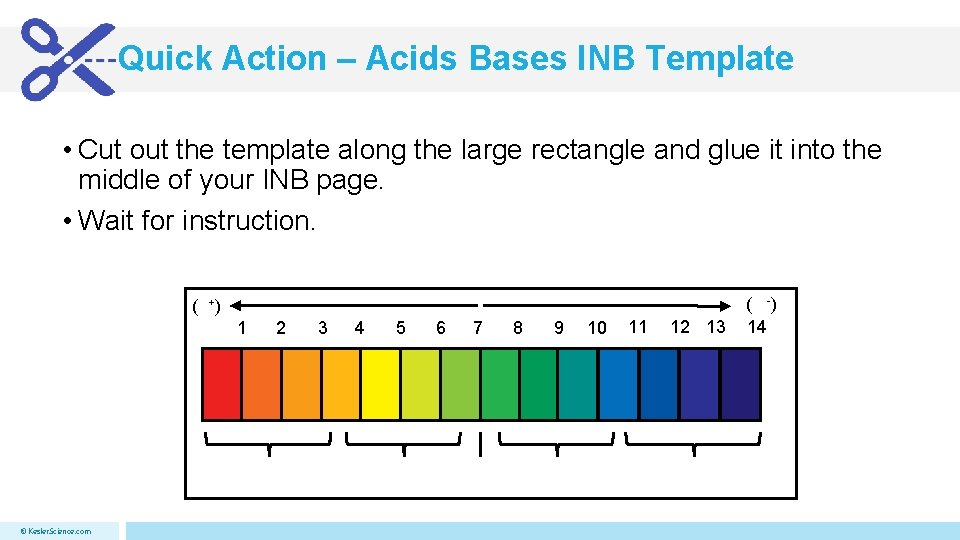
Quick Action – Acids Bases INB Template • Cut out the template along the large rectangle and glue it into the middle of your INB page. • Wait for instruction. ( +) 1 © Kesler. Science. com 2 3 4 5 6 7 8 9 10 11 12 13 ( -) 14
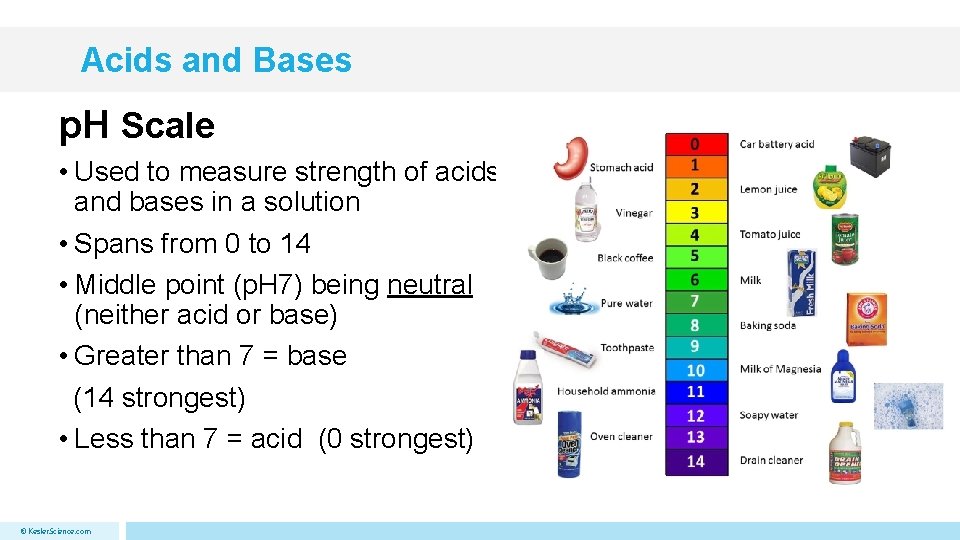
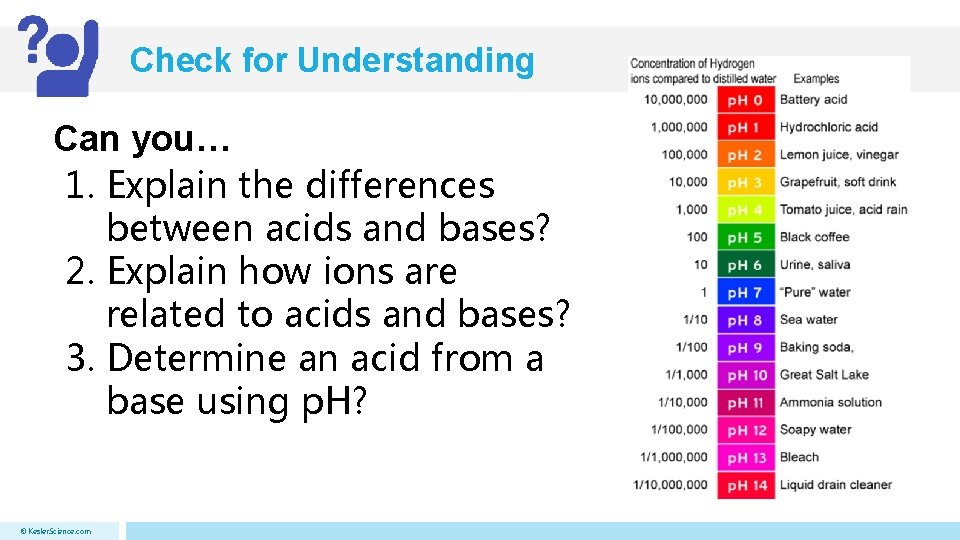
Acids and Bases p. H Scale • Used to measure strength of acids and bases in a solution • Spans from 0 to 14 • Middle point (p. H 7) being neutral (neither acid or base) • Greater than 7 = base (14 strongest) • Less than 7 = acid (0 strongest) © Kesler. Science. com
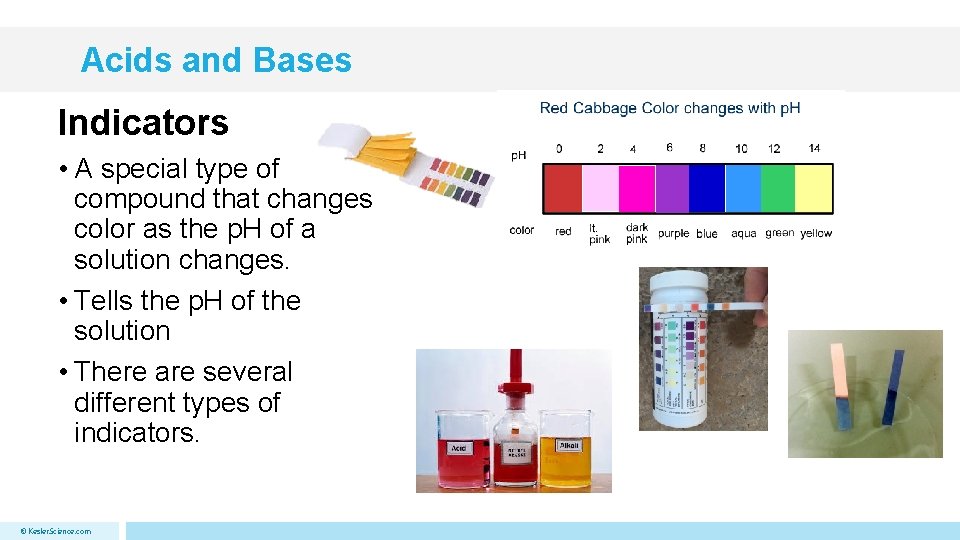
Acids and Bases Indicators • A special type of compound that changes color as the p. H of a solution changes. • Tells the p. H of the solution • There are several different types of indicators. © Kesler. Science. com
Quick Action – Acids and Bases Practice Get with a partner and talk about p. H indicators. Where have you seen indicators used? © Kesler. Science. com
Check for Understanding Can you… 1. Explain the differences between acids and bases? 2. Explain how ions are related to acids and bases? 3. Determine an acid from a base using p. H? © Kesler. Science. com
- Slides: 20