Acids and Bases OVERVIEW 1 Definitions and properties

Acids and Bases

OVERVIEW 1. Definitions and properties of acids and bases 2. Naming structure for acids 3. Neutralization 4. Anhydrides 5. Strong & weak acids and bases 6. Titration
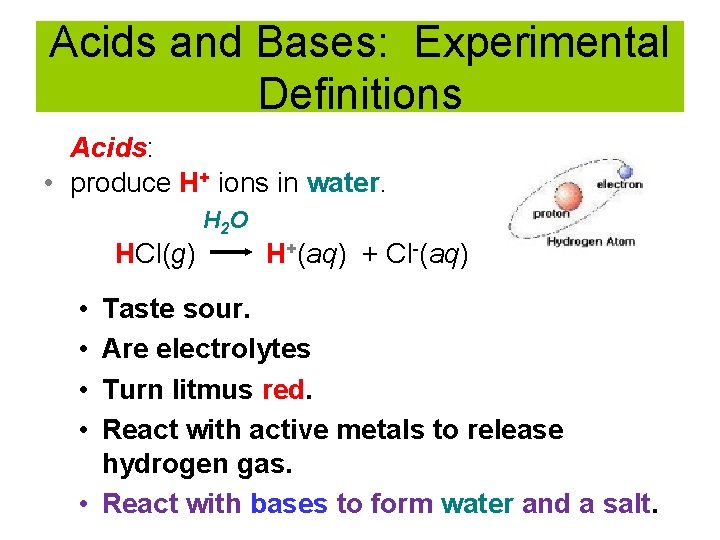
Acids and Bases: Experimental Definitions Acids: • produce H+ ions in water. H 2 O HCl(g) • • H+(aq) + Cl-(aq) Taste sour. Are electrolytes Turn litmus red. React with active metals to release hydrogen gas. • React with bases to form water and a salt.
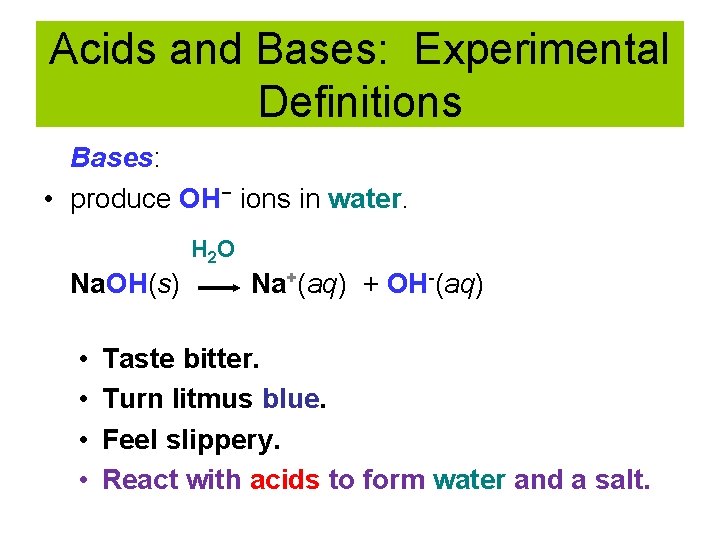
Acids and Bases: Experimental Definitions Bases: • produce OH− ions in water. H 2 O Na. OH(s) • • Na+(aq) + OH-(aq) Taste bitter. Turn litmus blue. Feel slippery. React with acids to form water and a salt.

Acids and Bases: Experimental Definitions
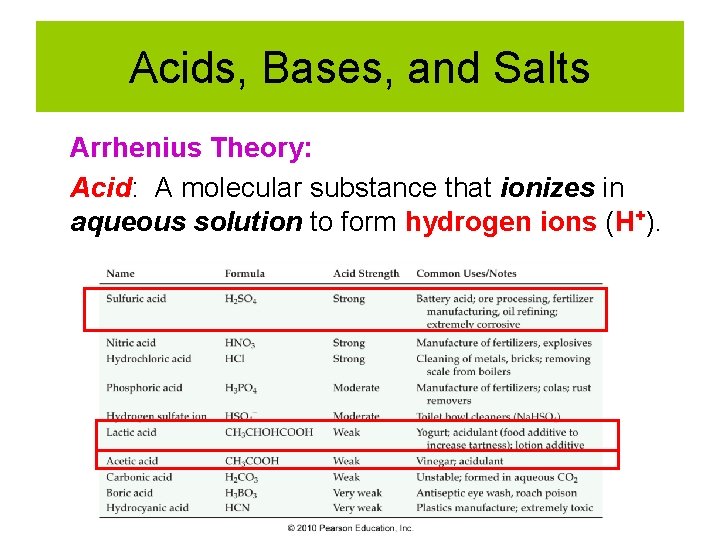
Acids, Bases, and Salts Arrhenius Theory: Acid: A molecular substance that ionizes in aqueous solution to form hydrogen ions (H+).
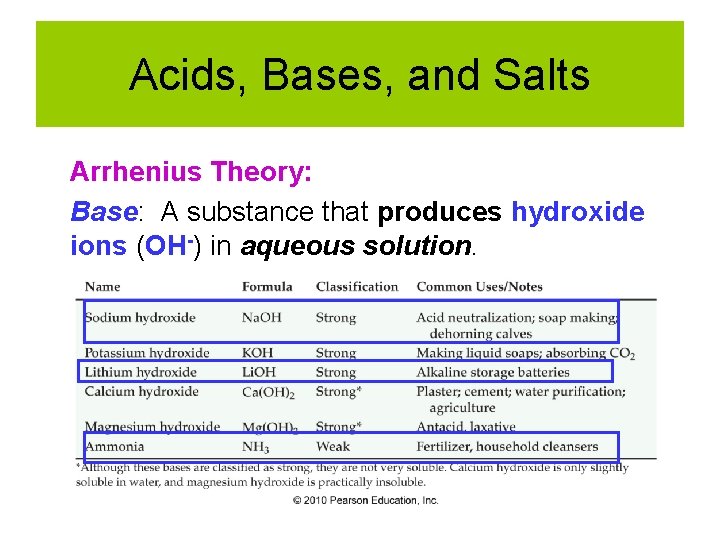
Acids, Bases, and Salts Arrhenius Theory: Base: A substance that produces hydroxide ions (OH-) in aqueous solution.
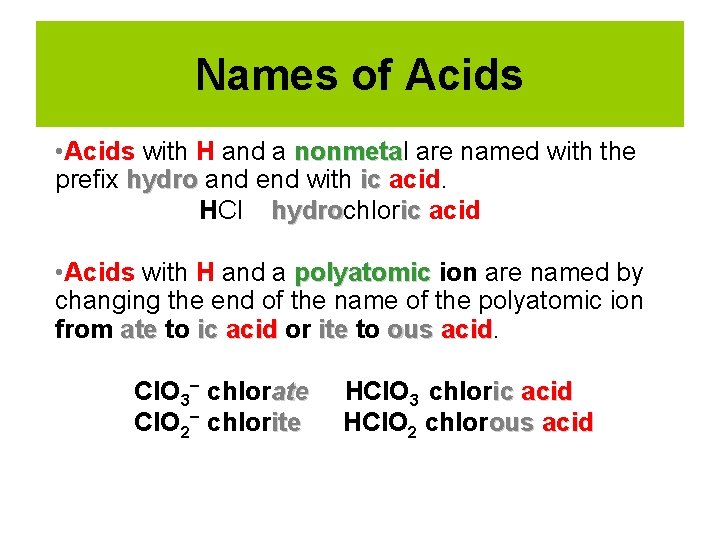
Names of Acids • Acids with H and a nonmetal nonmeta are named with the prefix hydro and end with ic acid. HCl hydrochlor ic acid hydro • Acids with H and a polyatomic ion are named by changing the end of the name of the polyatomic ion from ate to ic acid or ite to ous acid Cl. O 3− chlorate Cl. O 2− chlorite HCl. O 3 chloric acid HCl. O 2 chlorous acid
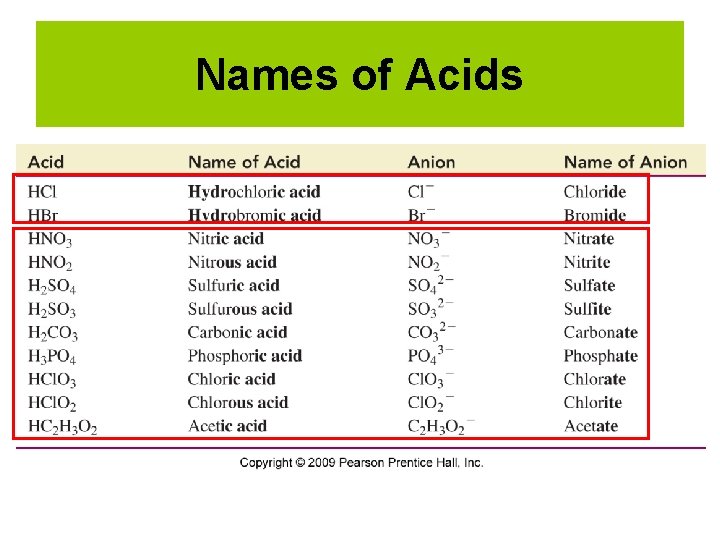
Names of Acids
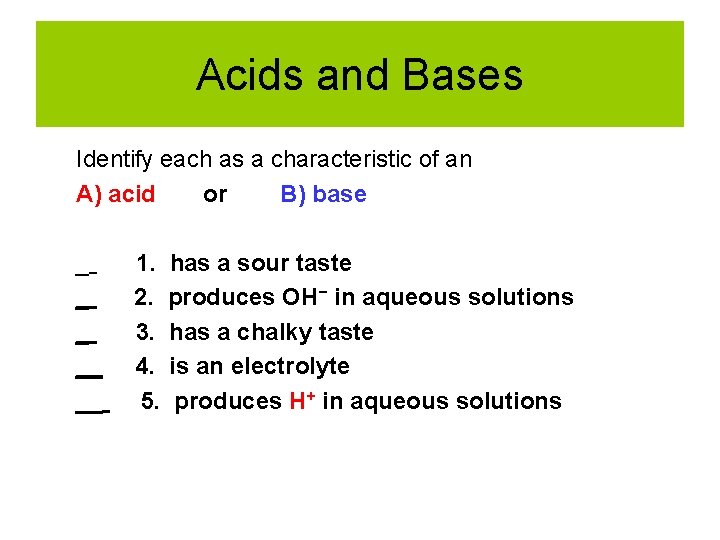
Acids and Bases Identify each as a characteristic of an A) acid or B) base _ __ __ 1. has a sour taste 2. produces OH− in aqueous solutions 3. has a chalky taste 4. is an electrolyte 5. produces H+ in aqueous solutions
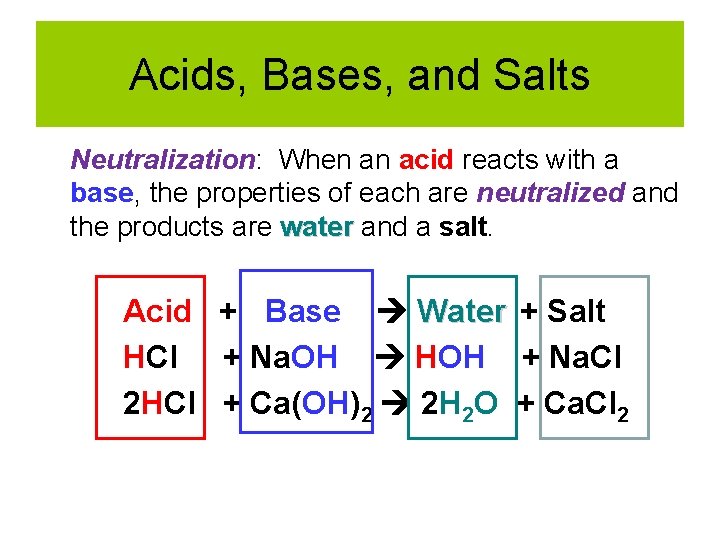
Acids, Bases, and Salts Neutralization: When an acid reacts with a base, the properties of each are neutralized and the products are water and a salt. Acid + Base Water + Salt HCl + Na. OH HOH + Na. Cl 2 HCl + Ca(OH)2 2 H 2 O + Ca. Cl 2
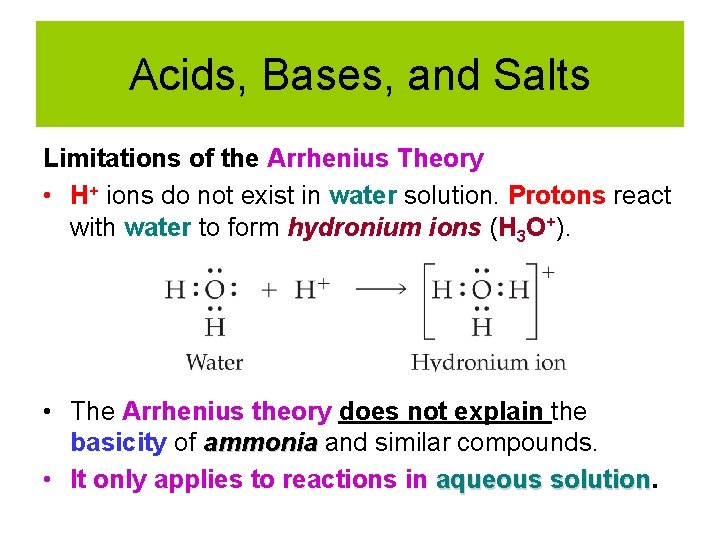
Acids, Bases, and Salts Limitations of the Arrhenius Theory • H+ ions do not exist in water solution. Protons react with water to form hydronium ions (H 3 O+). • The Arrhenius theory does not explain the basicity of ammonia and similar compounds. • It only applies to reactions in aqueous solution
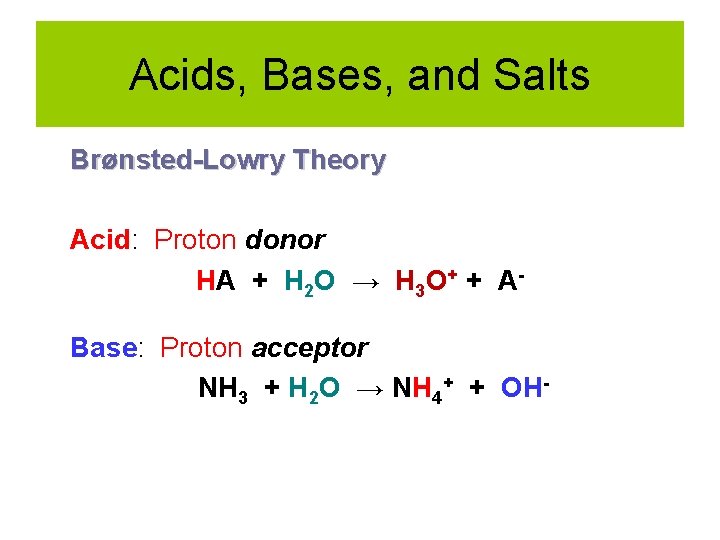
Acids, Bases, and Salts Brønsted-Lowry Theory Acid: Proton donor HA + H 2 O → H 3 O+ + ABase: Proton acceptor NH 3 + H 2 O → NH 4+ + OH-
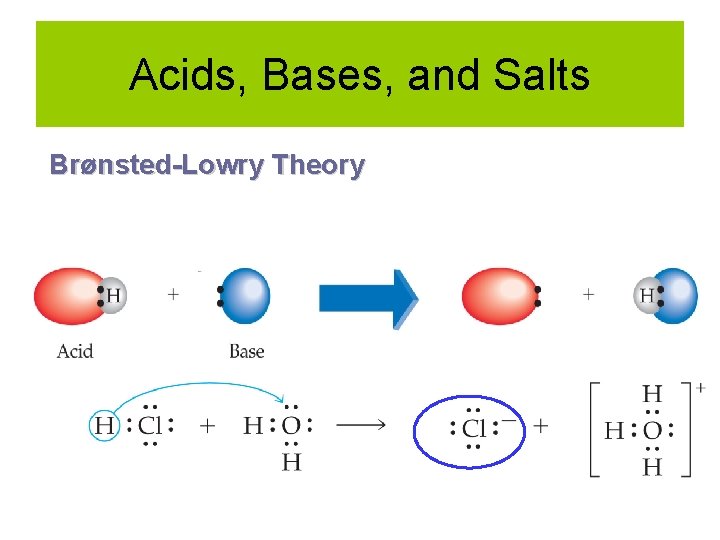
Acids, Bases, and Salts Brønsted-Lowry Theory
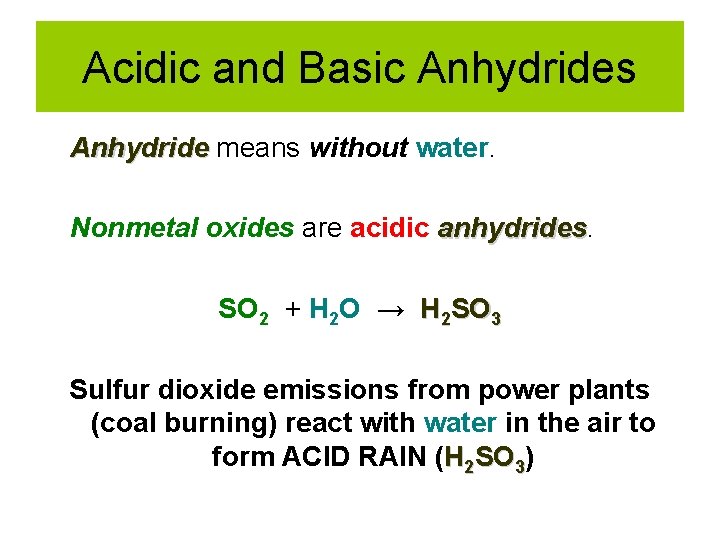
Acidic and Basic Anhydrides Anhydride means without water. Nonmetal oxides are acidic anhydrides SO 2 + H 2 O → H 2 SO 3 Sulfur dioxide emissions from power plants (coal burning) react with water in the air to form ACID RAIN (H 2 SO 3)
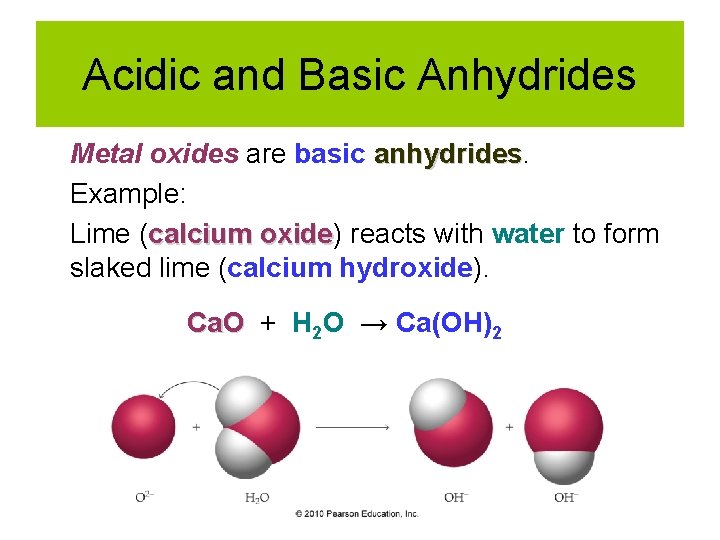
Acidic and Basic Anhydrides Metal oxides are basic anhydrides Example: Lime (calcium oxide) oxide reacts with water to form slaked lime (calcium hydroxide). Ca. O + H 2 O → Ca(OH)2
Acidic and Basic Anhydrides Ca. O SO 2
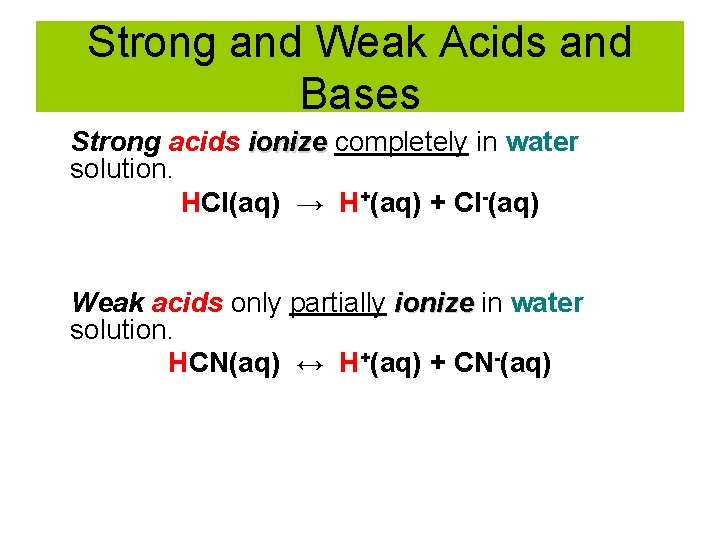
Strong and Weak Acids and Bases Strong acids ionize completely in water solution. HCl(aq) → H+(aq) + Cl-(aq) Weak acids only partially ionize in water solution. HCN(aq) ↔ H+(aq) + CN-(aq)

Strong and Weak Acids and Bases Strong bases ionize completely in water solution. H 2 O Na. OH(s) → Na+(aq) + OH-(aq) Weak bases only partially ionize in water solution. NH 3(g) + H 2 O ↔ NH 4+(aq) + OH-(aq)
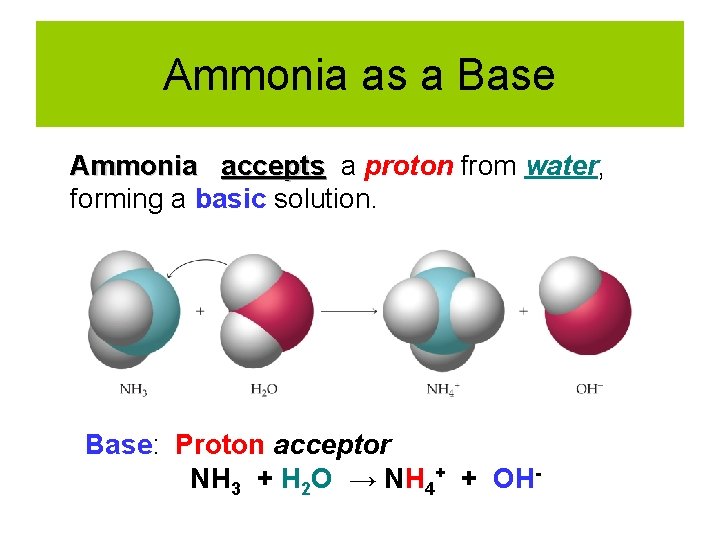
Ammonia as a Base Ammonia accepts a proton from water, forming a basic solution. Base: Proton acceptor NH 3 + H 2 O → NH 4+ + OH-
Neutralization The reaction of an acid with a base is called neutralization. Water molecules are the result of the reaction between hydrogen ions and hydroxide ions. H+ + OH- ↔ HOH
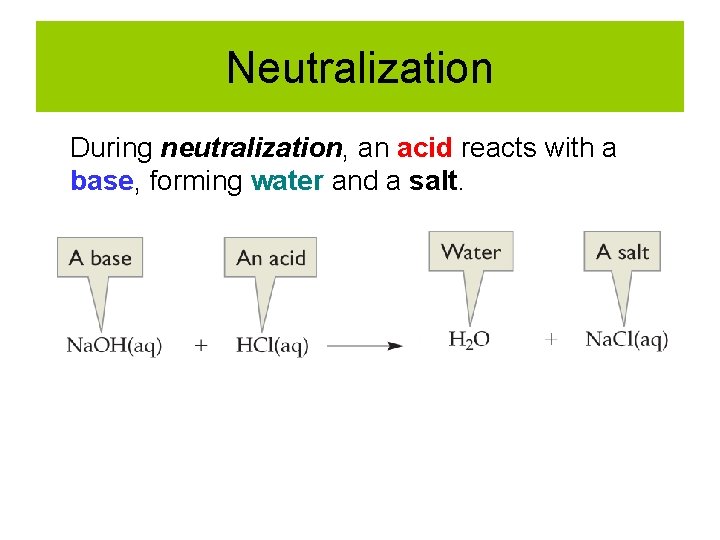
Neutralization During neutralization, an acid reacts with a base, forming water and a salt.
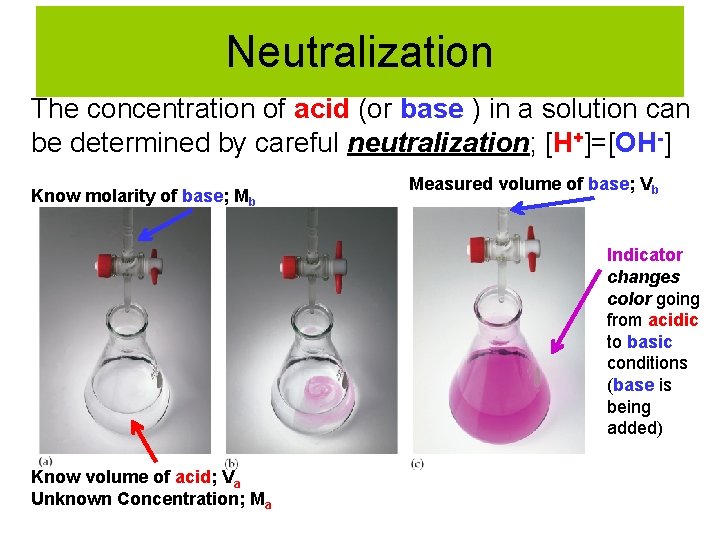
Neutralization The concentration of acid (or base ) in a solution can be determined by careful neutralization; [H+]=[OH-] Know molarity of base; Mb Measured volume of base; Vb Indicator changes color going from acidic to basic conditions (base is being added) Know volume of acid; Va Unknown Concentration; Ma
![Titration Phenolphthalein changes color basic Equivalence point [H+] = [OH-] acidic Titration Phenolphthalein changes color basic Equivalence point [H+] = [OH-] acidic](http://slidetodoc.com/presentation_image_h2/b0df7bf060721f7ba26e637b1b61b506/image-24.jpg)
Titration Phenolphthalein changes color basic Equivalence point [H+] = [OH-] acidic
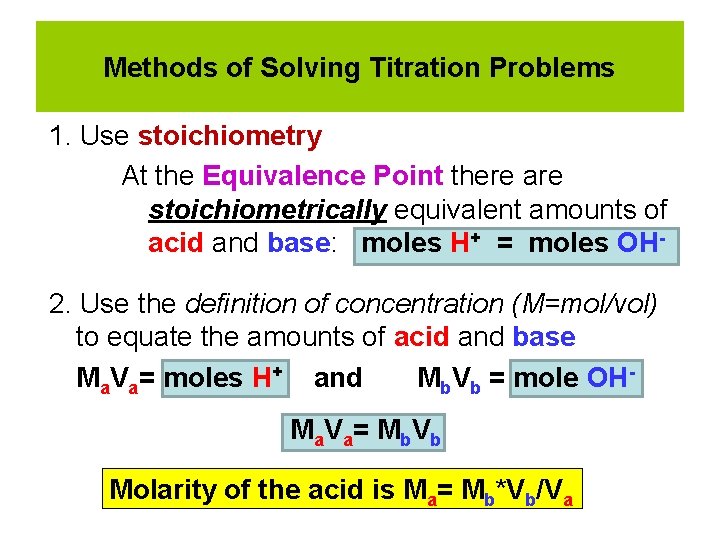
Methods of Solving Titration Problems 1. Use stoichiometry At the Equivalence Point there are stoichiometrically equivalent amounts of acid and base: moles H+ = moles OH 2. Use the definition of concentration (M=mol/vol) to equate the amounts of acid and base Ma. Va= moles H+ and Mb. Vb = mole OHMa V a = M b V b Molarity of the acid is Ma= Mb*Vb/Va
![Titration Phenolphthalein changes color Equivalence point [H+] = [OH-] Molarity of the acid is Titration Phenolphthalein changes color Equivalence point [H+] = [OH-] Molarity of the acid is](http://slidetodoc.com/presentation_image_h2/b0df7bf060721f7ba26e637b1b61b506/image-26.jpg)
Titration Phenolphthalein changes color Equivalence point [H+] = [OH-] Molarity of the acid is Ma= Mb*Vb/Va
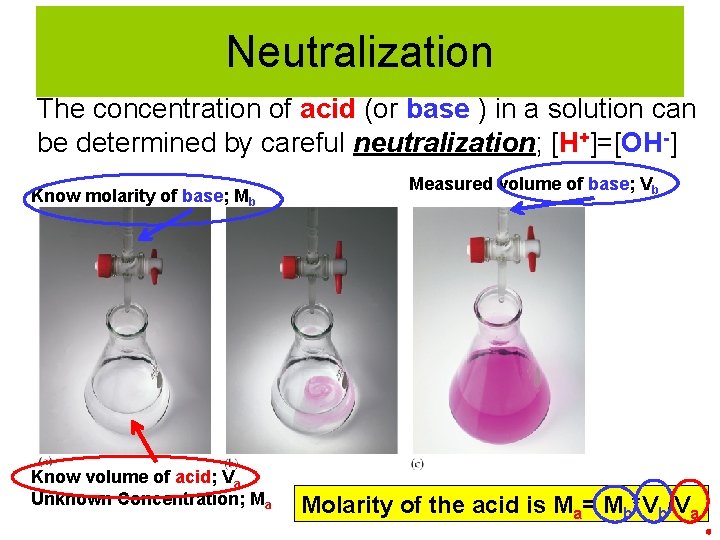
Neutralization The concentration of acid (or base ) in a solution can be determined by careful neutralization; [H+]=[OH-] Know molarity of base; Mb Know volume of acid; Va Unknown Concentration; Ma Measured volume of base; Vb Molarity of the acid is Ma= Mb*Vb/Va
Summary 1. Acids: produce H+ ions in water; Bases: produce OH− ions in water. (Arrhenius Theory) 2. Acid: Proton donor; Base: Proton acceptor (Brønsted-Lowry Theory) 3. Neutralization: When an acid reacts with a base; the products are water and a salt 4. H+ ions do not exist in water solution. Protons react with water to form hydronium ions (H 3 O+). 5. Anhydride means without water. Nonmetal oxides are acidic anhydrides Metal oxides are basic anhydrides 6. Strong acids/ bases ionize completely in water solution. Weak acids / bases only partially ionize in water solution. 7. With titration, titration the concentration of an unknown acid or base can be found using stoichiometry ([H+]=[OH-]) and the definition of concentration (M= mol/L)
- Slides: 28