ACIDS AND BASES NOTES Chapter 25 ACIDS Donates

ACIDS AND BASES NOTES Chapter 25

ACIDS �Donates an H+ (HYDROGEN ION), also called a proton donor. Taste SOUR (Think orange juice or grapefruit juice) p. H is less than 7 Corrosive to METALS Changes litmus RED

ACIDS �Becomes less acidic when mixed with a BASE Identifying an Acid Acts as a proton donor Will demonstrate any of the above characteristics Chemical formula begins with H-

NAMING ACIDS �Compounds that begin with H- (it is really an H+1 ion) are probably acids Example: HCl = hydrochloric acid H 2 SO 4 = sulfuric acid
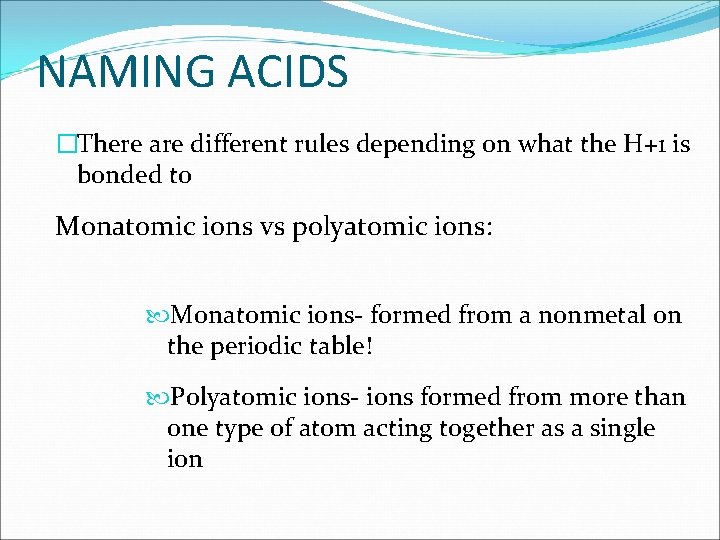
NAMING ACIDS �There are different rules depending on what the H+1 is bonded to Monatomic ions vs polyatomic ions: Monatomic ions- formed from a nonmetal on the periodic table! Polyatomic ions- ions formed from more than one type of atom acting together as a single ion
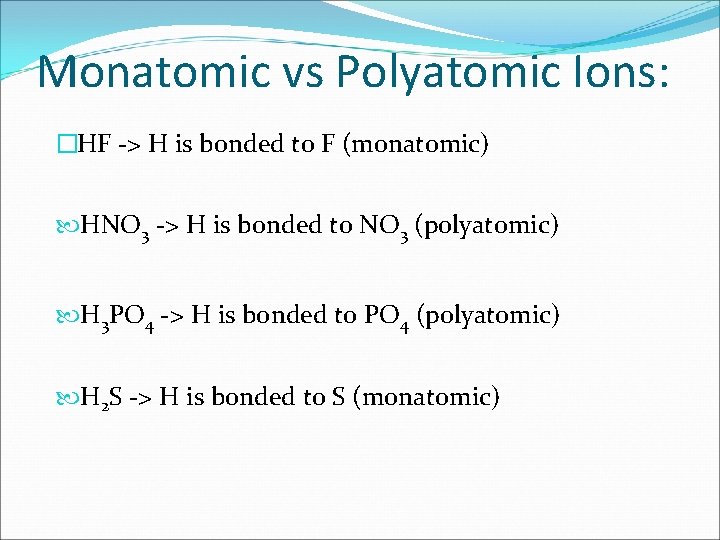
Monatomic vs Polyatomic Ions: �HF -> H is bonded to F (monatomic) HNO 3 -> H is bonded to NO 3 (polyatomic) H 3 PO 4 -> H is bonded to PO 4 (polyatomic) H 2 S -> H is bonded to S (monatomic)

RULES FOR MONATOMIC: �Use “HYDRO-” prefix • Use “-ic” suffix • End with “acid”
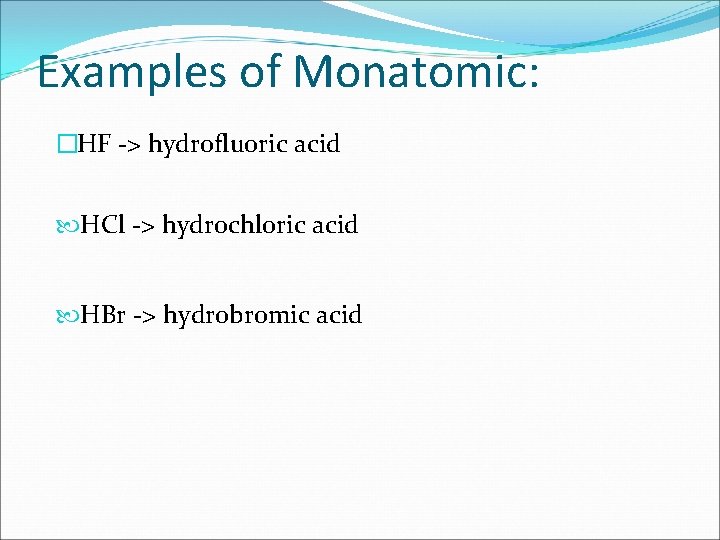
Examples of Monatomic: �HF -> hydrofluoric acid HCl -> hydrochloric acid HBr -> hydrobromic acid

RULES FOR POLYATOMIC: �We need our lists!!! • Do NOT use “hydro-” • Use “-ic” suffix for ions that end with “-ate” • Use “-ous” suffix for ions that end with “-ite” • End with “acid”
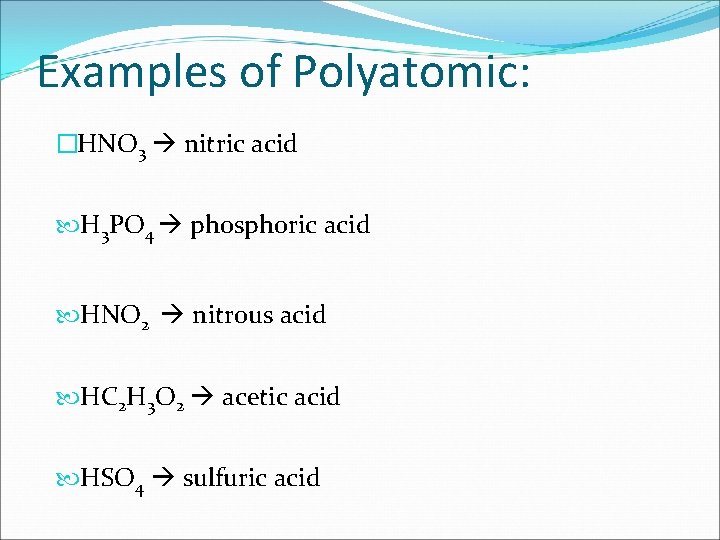
Examples of Polyatomic: �HNO 3 nitric acid H 3 PO 4 phosphoric acid HNO 2 nitrous acid HC 2 H 3 O 2 acetic acid HSO 4 sulfuric acid
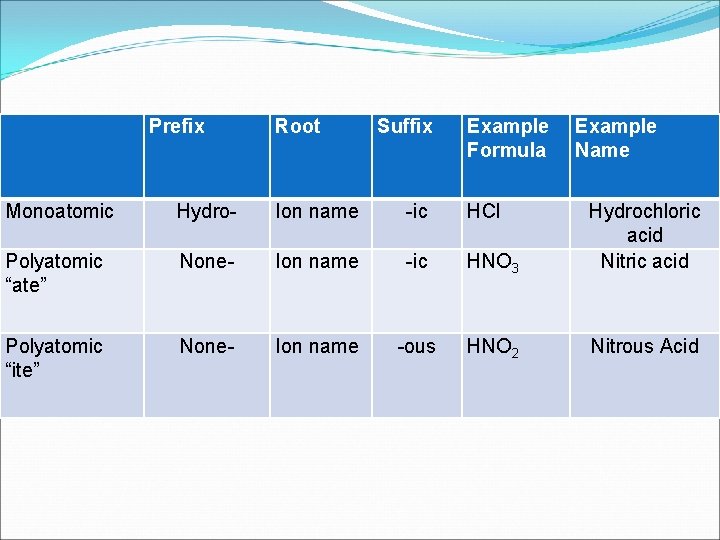
Prefix Root Suffix Example Formula Example Name Monoatomic Hydro- Ion name -ic HCl Polyatomic “ate” None- Ion name -ic HNO 3 Hydrochloric acid Nitric acid Polyatomic “ite” None- Ion name -ous HNO 2 Nitrous Acid

EXCEPTIONS: �Polyatomic ions that have names that end in “-ide” �Should be named as monatomic ions!

BASES: �Accepts a H+ ion, also called a proton acceptor p. H values of greater than 7 Feels slippery (Dissolving the oils and fats in your skin!) Changes litmus BLUE Becomes less basic when mixed with an acid

IDENTIFYING A BASE: �Will act as a proton acceptor Will demonstrate any of the above characteristics Will have a chemical formula that includes the hydroxide ion (OH ) Such as Na. OH (sodium hydroxide)
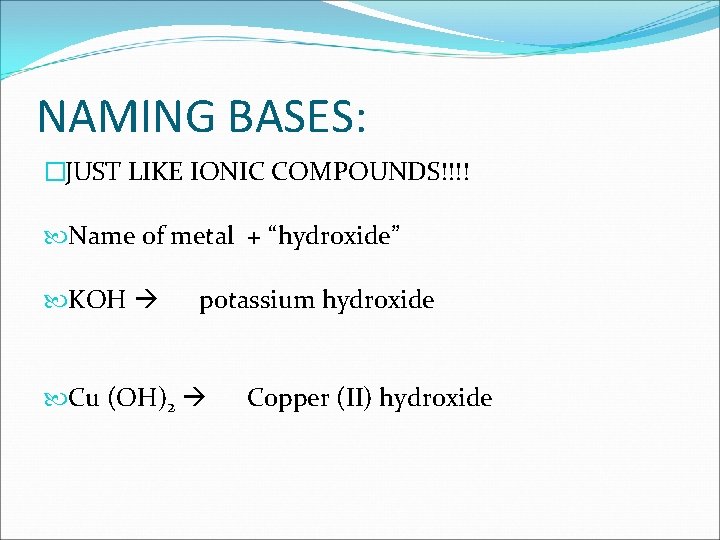
NAMING BASES: �JUST LIKE IONIC COMPOUNDS!!!! Name of metal + “hydroxide” KOH potassium hydroxide Cu (OH)2 Copper (II) hydroxide
- Slides: 15