Acids and Bases Naming Acids Binary Acids contains

Acids and Bases

Naming Acids Binary Acids (contains H & 1 other element) • Hydro- and –ic if anion does not contain oxygen HCl-hydrochloric acid Oxyacids (contain H, O, & other elements) • -ic anion ends in –ate H 2 SO 4 - SO 4 is sulfate = sulfuric acid • -ous anion ends in -ite H 2 SO 3 -SO 3 is sulfite = sulfurous acid

Common Acids • • • Sour milk (lactic acid) Vinegar (acetic acid) Carbonated beverages (phosphoric acid) Lemons, oranges (citric acid) Apples (malic acid) Grape Juice (tartaric acid)

Sulfuric Acid • Most commonly used product in the world • Petroleum • Automobile batteries • Dehydration agent • Metallurgy • Refining

Nitric Acid • • Stains proteins yellow Suffocating odor Stains skin Causes burns • • • Explosives Rubber Plastics Dyes Pharmaceuticals

Phosphoric Acid • Manufacturing fertilizers for plants and animal feed • Flavoring agent in beverages • Cleaning agent for dairy equipment

Hydrochloric acid • Produced in the stomach • Cleaning agent • Maintain acidity in pools

Common Properties of Acids • • Sour taste Produce hydronium H 3 O+ ions Change the color of acid-base indicators React with active metals to release hydrogen gas • React with bases to produce salts and water • Conduct electric current

Bases

Common Bases • Lye (sodium hydroxide) • Milk of magnesia (magnesium hydroxide) • Antacids (aluminum hydroxide)

Common Properties of Bases • • • Taste bitter Produce hydroxide OH- ions Change the color of acid-base indicators Feel slippery React with acids to produce salts and water Conduct electric current

Specific Definitions of Acids/Bases

Arrhenius Acids and Bases • Arrhenius acid-increases H+ ions (H 3 O+) H 2 SO 4 + H 2 O H 3 O+ +HSO 4 • Arrhenius base-increases OH- ions Ca(OH)2 Ca+2 + 2 OH-

Weak/Strong • Weak acid-weak electrolyte (end in -COOH) • Strong acid-ionizes completely, strong electrolyte (HCl) • Alkaline-base completely dissociates in water to yield OH- ions Na. OH Na+ + OH • Strong bases-completely dissociates, strong electrolyte (end in OH)

Bronsted-Lowry • Bronsted-Lowry Acid-proton donor • Bronsted-Lowry Base-proton acceptor HCl + NH 3 NH 4+ + Cl • Monoprotic acid-donates one proton HCl • Diprotic acid-donates two protons H 2 SO 4 • Triprotic acid-donates three protons H 3 PO 4
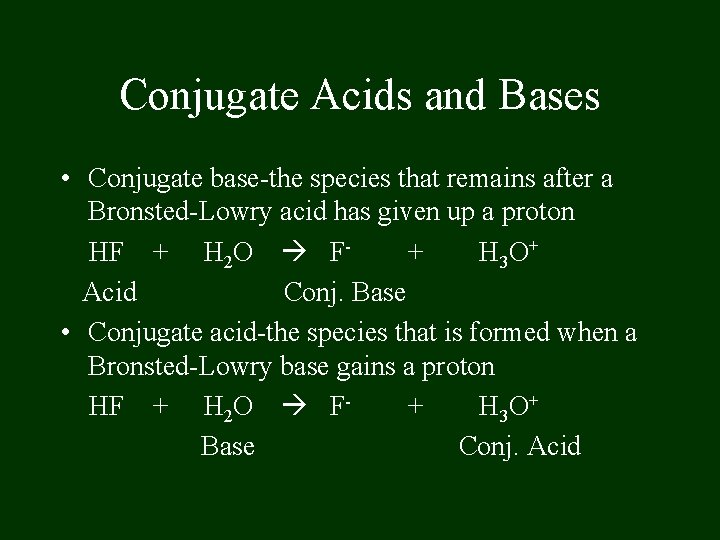
Conjugate Acids and Bases • Conjugate base-the species that remains after a Bronsted-Lowry acid has given up a proton HF + H 2 O F+ H 3 O+ Acid Conj. Base • Conjugate acid-the species that is formed when a Bronsted-Lowry base gains a proton HF + H 2 O F+ H 3 O+ Base Conj. Acid

Strength of Acid/Conj. Base Strong acid weak conjugate base Weak acid strong conjugate base

Other Terms • Amphoteric compound-any species that can react as either and acid or a base • Neutralization-the reaction of hydronium ions and hydroxide ions to form water molecules and salt Self-ionization of water H 3 O+ + OH- 2 H 2 O or H 20 + H 2 O H 3 O+ + OH • Salt-an ionic compound composed of a cation from a base and an anion from an acid

Buffers • A solution that resists a change in its p. H even when a strong acid or base is added to it. • A solution is buffered by the presence of a weak acid and its conjugate base. • Added H+ reacts with base • Added OH- reacts with weak acid

Titration Practice Problems

Ex 1 A 30. m. L volume of HCl is titrated with 23 m. L of 0. 20 M Na. OH. What is the molarity of HCl in this solution?

Answer 0. 15 M

Ex 2 A 26 m. L volume of Na. OH is titrated with 23 m. L of 0. 20 MHCl. What is the molarity of Na. OH in this solution?

Answer 0. 18 M

Ex 3 A 40. m. L volume of H 2 SO 4 is titrated with 38 m. L of 0. 24 M Na. OH. What is the molarity of H 2 SO 4 in this solution?

Answer 0. 11 M

Acid/Base Rhapsody

Is this the real life? Is this just fantasy? No protons around here. Hydronium reality. Open your book and take a look and seeeeeee…. I’m just a poor student – prof. give me your sympathy. Marks are easy come, they’re easy go. Sometimes they’re high, sometimes they’re low. As long as you understand it, it doesn’t really matter to me.

Mama, just had my class, Learned about Arrhenius, Bronsted Lowry, Lewis acids. Conjugate acid/base pairs. Amphoteric and hydrides pull my hair. Mama, oo-ooo-ooh, Some salts with hydrolize. If I don’t call again this time tomorrow. Carry on, carry on, I’m studying for this test.

Mama lots to learn acids strong and weak. Leveling affect- an A is what I seek. Acid-ionization constants-O, my brain is sore. Oxyacids, indicators change color! Mama ooooo. Water auto-ionizes forms hydroxide and hydronium all the time. What? ?

I see a little acid and its conjugate base Got a buffer! Will you do the calculation? ! Want a certain p. H, find the pka that’s near. Bronsted-Lowry, Bronsted-Lowry rules the day. O-o-ow… I’m just a poor student spent my money at dance clubs. He’s just a poor student studies titrations at home. Spare him this question of a polyprotic acid.

Easy come easy go-indicator tells you Now is the endpoint near I overshot again (and over) I think I got it now…. I got it now Oh. Oh Indicator, indicator lets me know The lab TA has a buret to decide for me, For MEEEEEEEEEEE!!!!!!

So you think you can do an acid/base titration!!! But when you overshoot the endpoint, it just leads to frustration. Oh, maybe, just a little more base maybe. I’ll think I’ll get it right. I got to get it right this time.
Doesn’t really matter-acid or base the same. Doesn’t really matter to meeeeee. Doesn’t really matter to me.
- Slides: 34