ACIDS AND BASES http www visionlearning comlibrarymoduleviewer php

ACIDS AND BASES http: //www. visionlearning. com/library/module_viewer. php? mid=58

ACIDS § The term acid comes from the Latin terms acere, which means “sour” and acidus, which means “sharp”. § Acids taste sour, are corrosive to metals, change litmus (a dye extracted from lichens) red, and become less acidic when mixed with bases. § Acids are compounds that contain hydrogen and can dissolve in water to release hydrogen (H+) ions into solution. H 20 + HCl H (aq) + Cl-(aq)


Uses of Acids § Acetic Acid = Vinegar § Citric Acid = lemons, limes, & oranges. It is in many sour candies such as lemonhead & sour patch. § Ascorbic acid = Vitamin C which your body needs to function. § Sulfuric acid is used in the production of fertilizers, steel, paints, and plastics. § Car batteries

BASES § Bases feel slippery, change litmus blue, and become less basic when mixed with acids. § Another word for base is ALKALI. Where have we heard that before? ? ? § Bases are substances that dissolve in water to release hydroxide ions (OH-) into solution. Na. OH H 20 Na+(aq) + OH-(aq)

Uses of Bases § Bases give soaps, ammonia, and many other cleaning products some of their useful properties. § The OH- ions interact strongly with certain substances, such as dirt and grease. § Chalk and oven cleaner are examples of familiar products that contain bases. § Your blood is a basic solution.

NEUTRALIZATION § As you can see from the equations, acids release H+ into solution and bases release OH-. If we were to mix an acid and base together, the H+ ion would combine with the OH- ion to make the molecule H 2 O, or plain water (neutral substance): H+(aq)+ OH-(aq) H 2 O
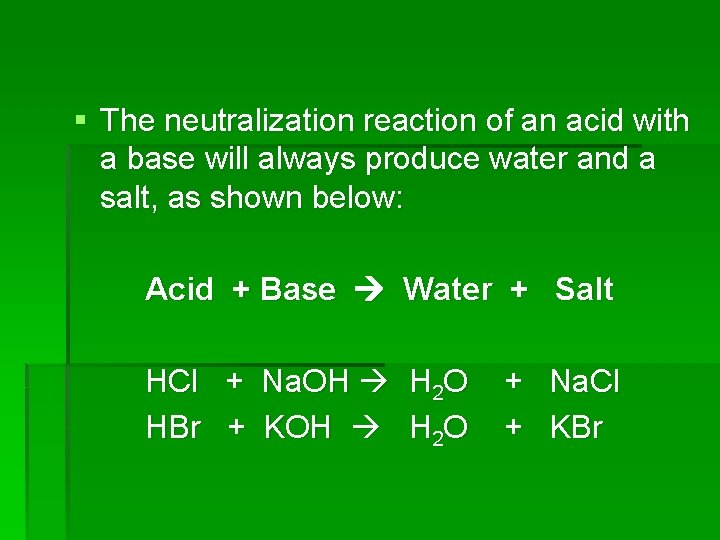
§ The neutralization reaction of an acid with a base will always produce water and a salt, as shown below: Acid + Base Water + Salt HCl + Na. OH H 2 O + Na. Cl HBr + KOH H 2 O + KBr


PREDICT: ACID OR BASE? Lemons/limes ACID
Pickles ACID

Baking soda (Na. HCO 3) BASE

Hand soap BASE

Ammonia (NH 3) BASE

Tums BASE

Coffee ACID

Apple juice ACID
Drain cleaner BASE

Tomatoes ACID

Glass cleaner BASE

Normal rain water SLIGHTLY ACIDIC

Water (H 2 O) NEUTRAL
How do we measure how acidic or basic something is? § Both acids and bases are related to the concentration of hydrogen ions present. § Acids increase the concentration of hydrogen ions, while bases decrease the concentration of hydrogen ions (by accepting them to form H 2 O). § The acidity or basicity of something therefore can be measured by its hydrogen ion concentration. § This is done with the p. H scale.
![The p. H scale is described by the formula: p. H = -log [H+] The p. H scale is described by the formula: p. H = -log [H+]](http://slidetodoc.com/presentation_image_h/e883f225b5923fdb5fefe5000592d9a5/image-24.jpg)
The p. H scale is described by the formula: p. H = -log [H+] Note: concentration is commonly abbreviated by using square brackets, thus, [H+] = hydrogen ion concentration. When measuring p. H, [H+] is in units of moles of H+ per liter of solution.
![What does THAT mean? For example, a solution with [H+] = 1 x 10 What does THAT mean? For example, a solution with [H+] = 1 x 10](http://slidetodoc.com/presentation_image_h/e883f225b5923fdb5fefe5000592d9a5/image-25.jpg)
What does THAT mean? For example, a solution with [H+] = 1 x 10 -7 moles/liter has a p. H equal to 7 (a simpler way to think about p. H is that it equals the exponent on the H+ concentration, ignoring the minus sign). [H+] = 1 x 10 -7 plugged into the equation is: -log [1 x 10 -7] = -log 10 [-7] = -(-7) = 7
Measuring p. H § The p. H scale ranges from 0 to 14. § Substances with a p. H between 0 and less than 7 are acids (p. H and [H+] are inversely related - lower p. H means higher [H+]). § Substances with a p. H greater than 7 and up to 14 are bases (higher p. H means lower [H+]). Right in the middle, at p. H = 7, are neutral substances, for example, pure water.
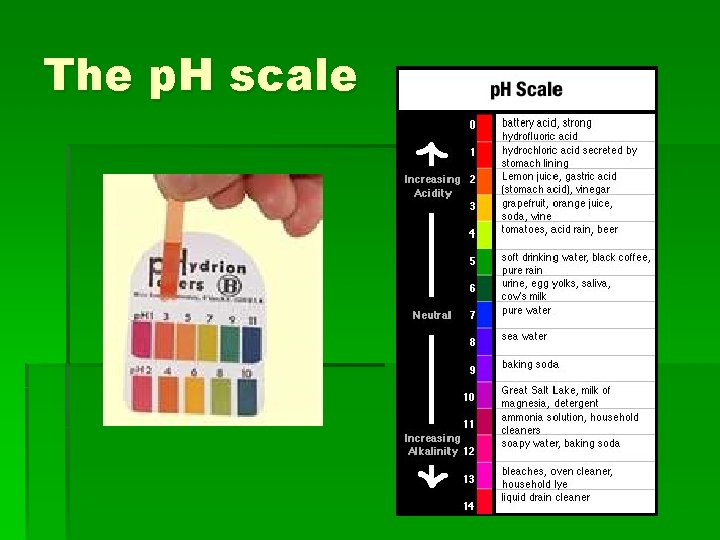
The p. H scale
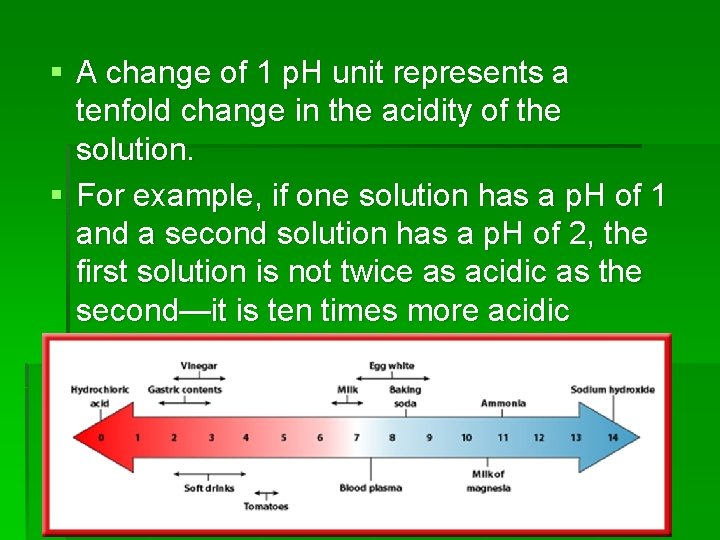
§ A change of 1 p. H unit represents a tenfold change in the acidity of the solution. § For example, if one solution has a p. H of 1 and a second solution has a p. H of 2, the first solution is not twice as acidic as the second—it is ten times more acidic
![What is the p. H? Is it acid/base/neutral? -log [1 x 10 -9] p. What is the p. H? Is it acid/base/neutral? -log [1 x 10 -9] p.](http://slidetodoc.com/presentation_image_h/e883f225b5923fdb5fefe5000592d9a5/image-29.jpg)
What is the p. H? Is it acid/base/neutral? -log [1 x 10 -9] p. H = 9 base -log [1 x 10 -7] p. H = 7 neutral [H+] = 1 x 10 -3 p. H = 3 acid [H+] = 1 x 10 -13 p. H = 13 base
- Slides: 29