Acids and Bases History of Acids and Bases

Acids and Bases

History of Acids and Bases In the early days of chemistry chemists were organizing physical and chemical properties of substances. They discovered that many substances could be placed in two different property categories: Substance B Substance A 1. Bitter taste 1. Sour taste 2. Reacts with carbonates to make CO 2 2. 3. Reacts with metals to produce H 2 3. Do not react with metals 4. Turns blue litmus pink 4. Turns red litmus blue 5. Reacts with B substances to make salt water 5. Reacts with A substances to make salt and water Reacts with fats to make soaps Arrhenius was the first person to suggest a reason why substances are in A or B due to their ionization in water.
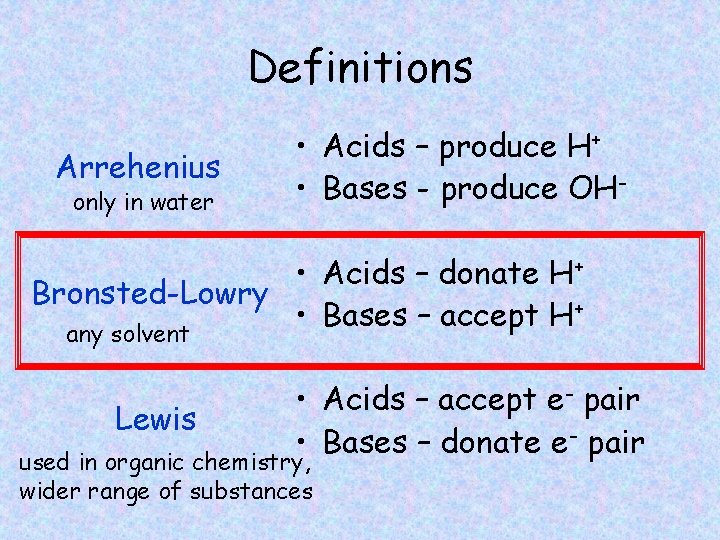
Definitions Arrehenius only in water • Acids – produce H+ • Bases - produce OH- • Acids – donate H+ Bronsted-Lowry • Bases – accept H+ any solvent Lewis • Acids – accept e- pair • Bases – donate e- pair used in organic chemistry, wider range of substances
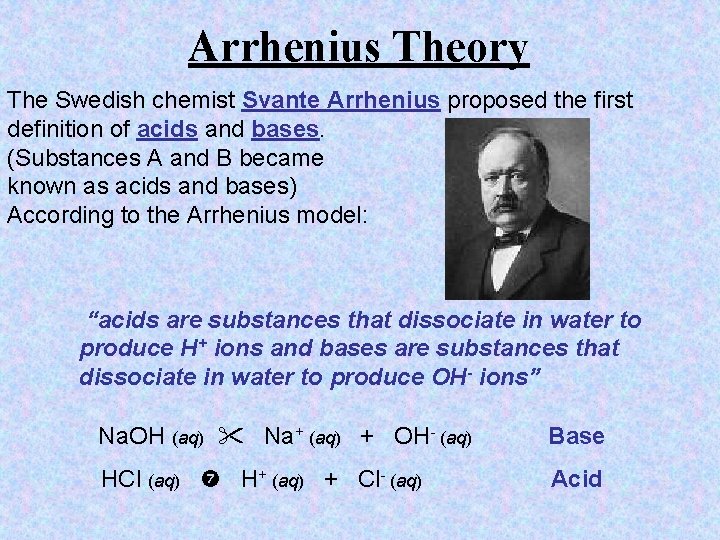
Arrhenius Theory The Swedish chemist Svante Arrhenius proposed the first definition of acids and bases. (Substances A and B became known as acids and bases) According to the Arrhenius model: “acids are substances that dissociate in water to produce H+ ions and bases are substances that dissociate in water to produce OH- ions” Na. OH (aq) Na+ (aq) + OH- (aq) Base HCl (aq) H+ (aq) + Cl- (aq) Acid
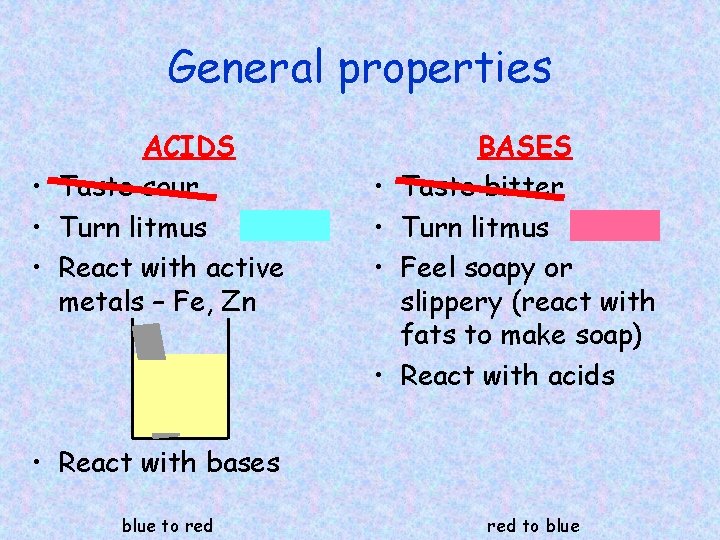
General properties ACIDS • Taste sour • Turn litmus • React with active metals – Fe, Zn • • BASES Taste bitter Turn litmus Feel soapy or slippery (react with fats to make soap) React with acids • React with bases blue to red to blue

What is an acid? • An acid is a solution that has an excess of H+ ions. It comes from the Latin word acidus that means "sharp" or "sour". • The more H + ions, the more acidic the solution.
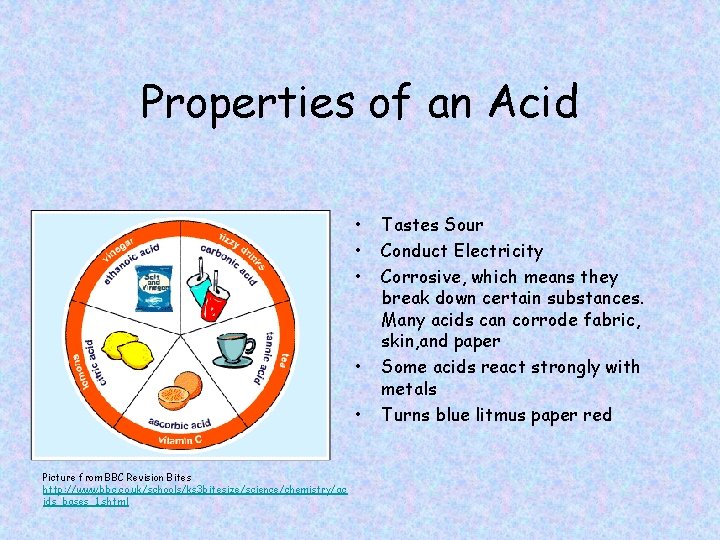
Properties of an Acid • • • Picture from BBC Revision Bites http: //www. bbc. co. uk/schools/ks 3 bitesize/science/chemistry/ac ids_bases_1. shtml Tastes Sour Conduct Electricity Corrosive, which means they break down certain substances. Many acids can corrode fabric, skin, and paper Some acids react strongly with metals Turns blue litmus paper red

Uses of Acids • Acetic Acid = Vinegar • Citric Acid = lemons, limes, & oranges. It is in many sour candies such as lemonhead & sour patch. • Ascorbic acid = Vitamin C which your body needs to function. • Sulfuric acid is used in the production of fertilizers, steel, paints, and plastics. • Car batteries

What is a base? • A base is a solution that has an excess of OHions. • Another word for base is alkali. • Bases are substances that can accept hydrogen ions

Properties of a Base • • Feel Slippery Taste Bitter Corrosive Can conduct electricity. (Think alkaline batteries. ) • Do not react with metals. • Turns red litmus paper blue.

Uses of Bases • Bases give soaps, ammonia, and many other cleaning products some of their useful properties. • The OH- ions interact strongly with certain substances, such as dirt and grease. • Chalk and oven cleaner are examples of familiar products that contain bases. • Your blood is a basic solution.

• Conjugate acid- compound formed when a base gains a hydrogen ion. • Conjugate base – compound formed when an acid loses a hydrogen ion.
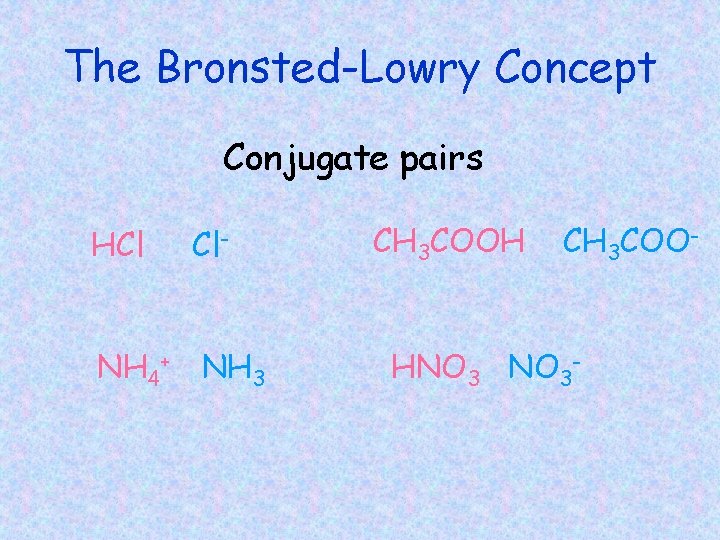
The Bronsted-Lowry Concept Conjugate pairs HCl Cl- NH 4+ NH 3 CH 3 COOH CH 3 COO- HNO 3 -
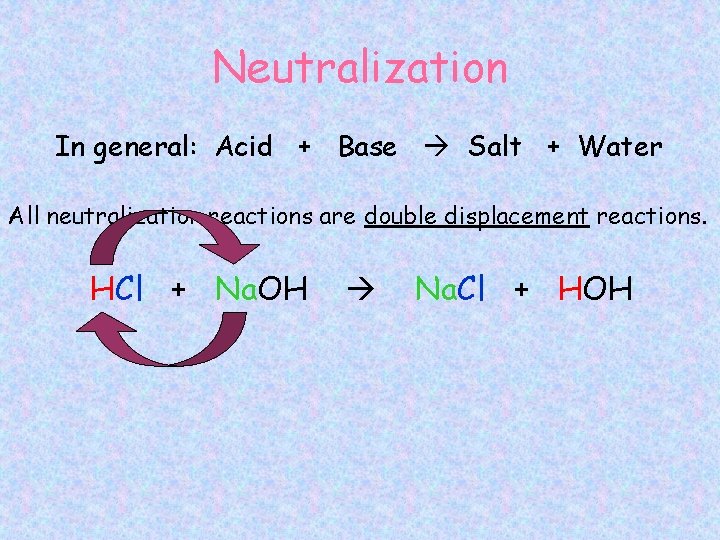
Neutralization In general: Acid + Base Salt + Water All neutralization reactions are double displacement reactions. HCl + Na. OH Na. Cl + HOH
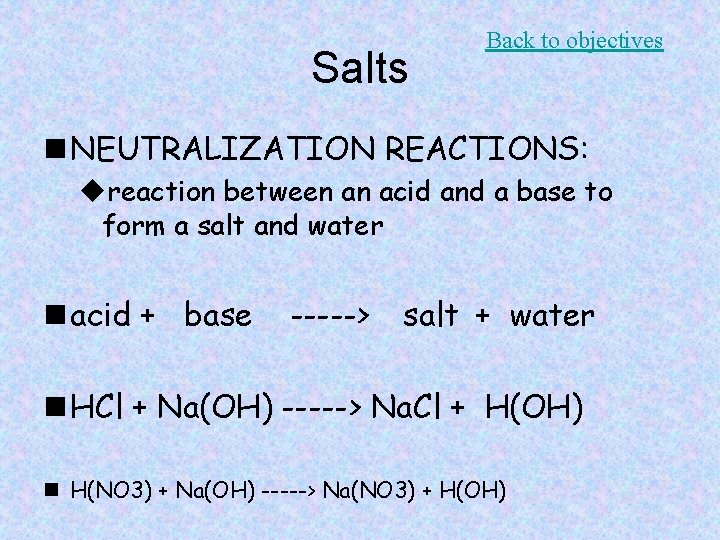
Salts Back to objectives n NEUTRALIZATION REACTIONS: ureaction between an acid and a base to form a salt and water n acid + base -----> salt + water n HCl + Na(OH) -----> Na. Cl + H(OH) n H(NO 3) + Na(OH) -----> Na(NO 3) + H(OH)
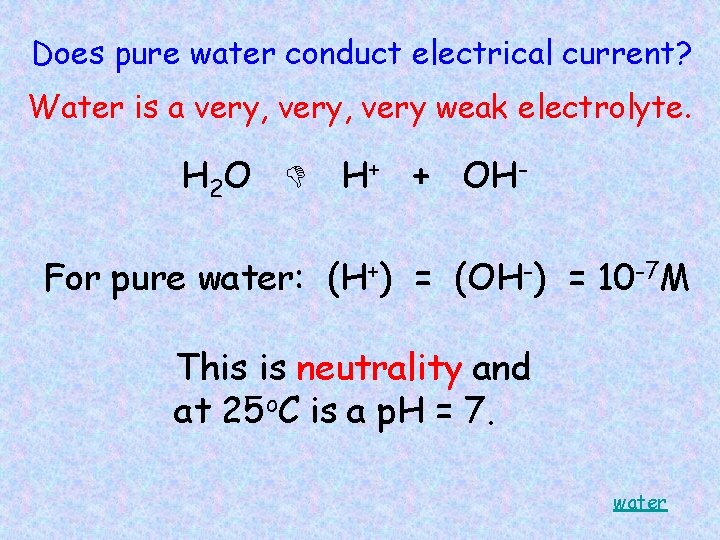
Does pure water conduct electrical current? Water is a very, very weak electrolyte. H 2 O H+ + OHFor pure water: (H+) = (OH-) = 10 -7 M This is neutrality and at 25 o. C is a p. H = 7. water
Let’s examine the behavior of an acid, HA, in aqueous solution. HA
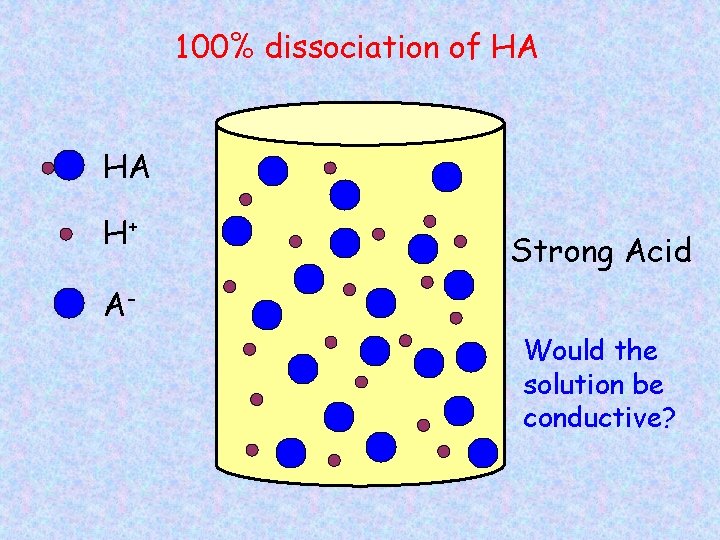
100% dissociation of HA HA H+ Strong Acid AWould the solution be conductive?
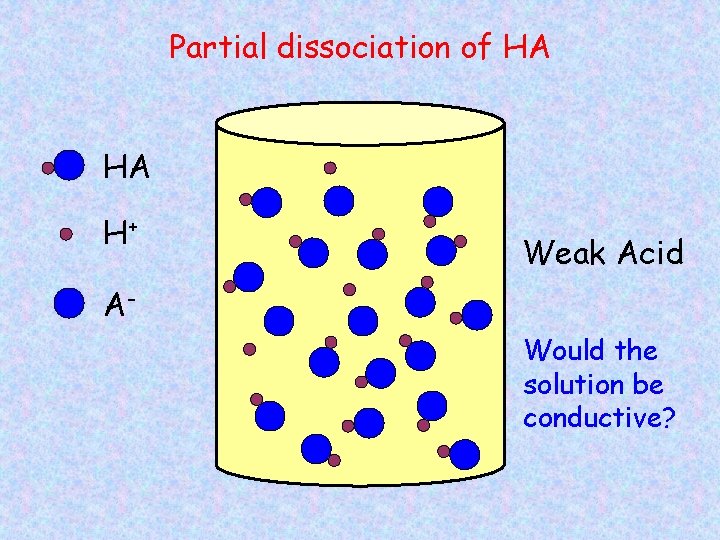
Partial dissociation of HA HA H+ Weak Acid AWould the solution be conductive?
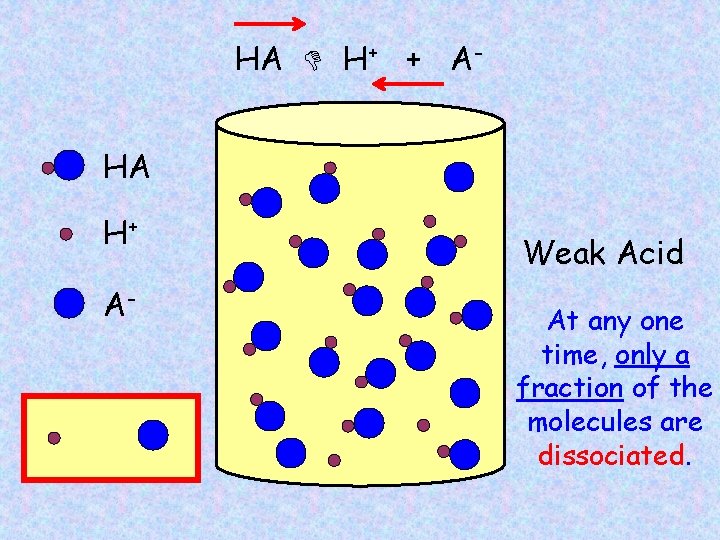
HA H+ + AHA H+ A- Weak Acid At any one time, only a fraction of the molecules are dissociated.

Strong and Weak Acids/Bases Strong acids/bases – 100% dissociation into ions HCl HNO 3 H 2 SO 4 Na. OH KOH Weak acids/bases – partial dissociation, both ions and molecules CH 3 COOH NH 3
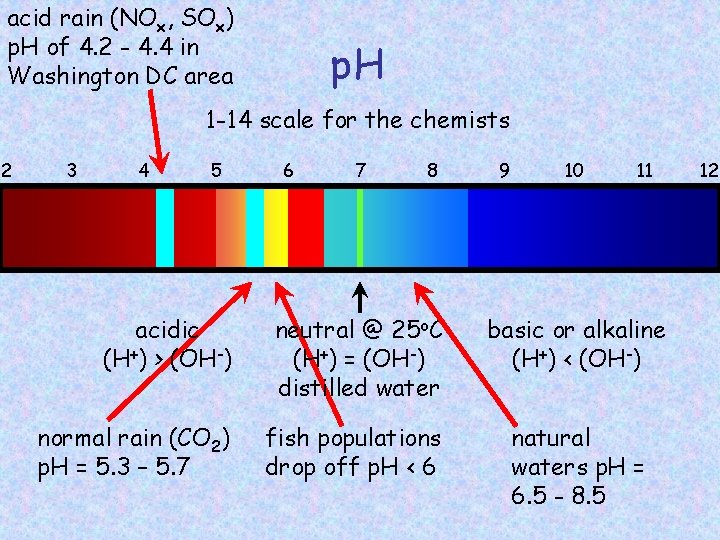
acid rain (NOx, SOx) p. H of 4. 2 - 4. 4 in Washington DC area p. H 1 -14 scale for the chemists 2 3 4 5 6 7 8 9 10 11 acidic (H+) > (OH-) neutral @ 25 o. C (H+) = (OH-) distilled water basic or alkaline (H+) < (OH-) normal rain (CO 2) p. H = 5. 3 – 5. 7 fish populations drop off p. H < 6 natural waters p. H = 6. 5 - 8. 5 12

Indicators • Show a color change to identify an acid or base • Types: – Litmus paper • Red – acid • Blue – base – Phenolphthalein (liquid) • Clear – acid, neutral • Pink – base

p. H • p. H SCALE: – Range of numbers from 0 to 14 – Indicates acid, base, and neutral along with strength
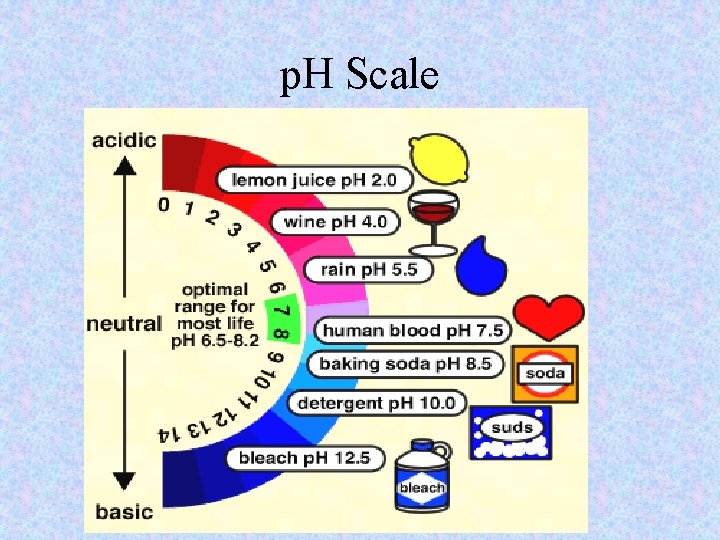
p. H Scale
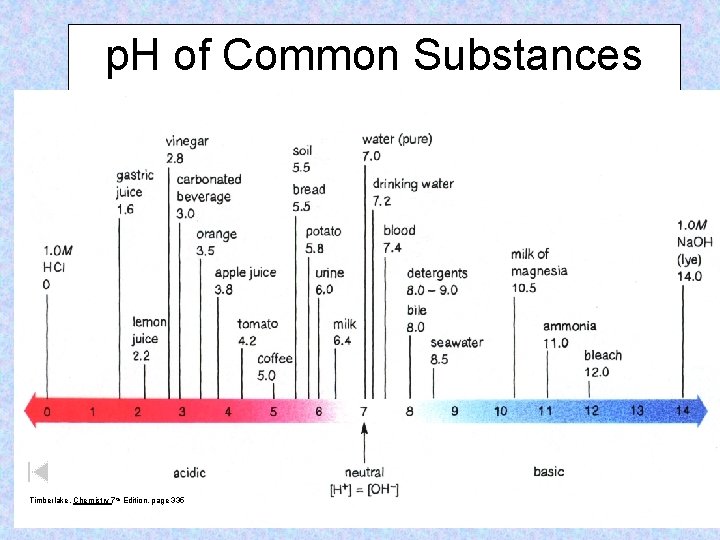
p. H of Common Substances Timberlake, Chemistry 7 th Edition, page 335
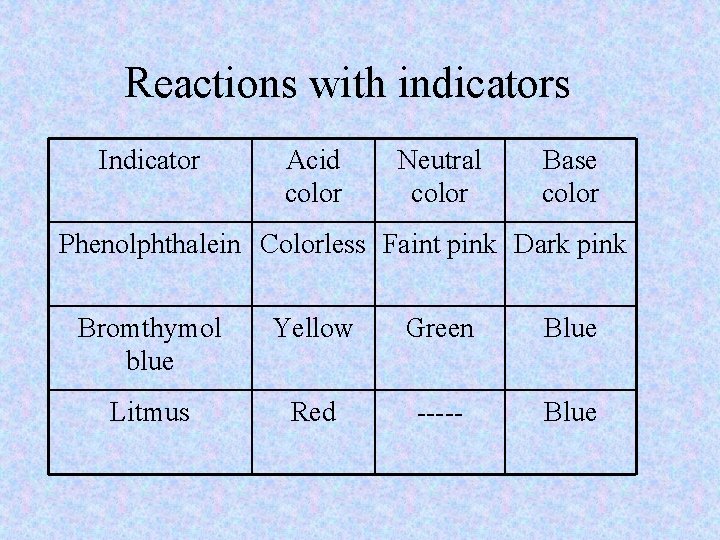
Reactions with indicators Indicator Acid color Neutral color Base color Phenolphthalein Colorless Faint pink Dark pink Bromthymol blue Yellow Green Blue Litmus Red ----- Blue

p. H paper • p. H paper changes color to indicate a specific p. H value.

What is an oxide? • An oxide is a compound of oxygen and another element. • Most oxides can be grouped into four types: – – acidic oxides basic oxides amphoteric oxides neutral oxides

Acidic oxides • Oxides of non-metal • Acidic oxides are often gases at room temperature.
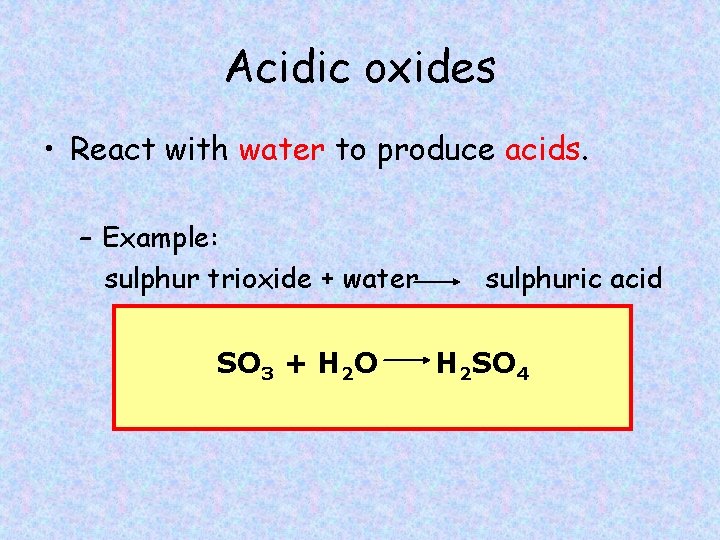
Acidic oxides • React with water to produce acids. – Example: sulphur trioxide + water SO 3 + H 2 O sulphuric acid H 2 SO 4
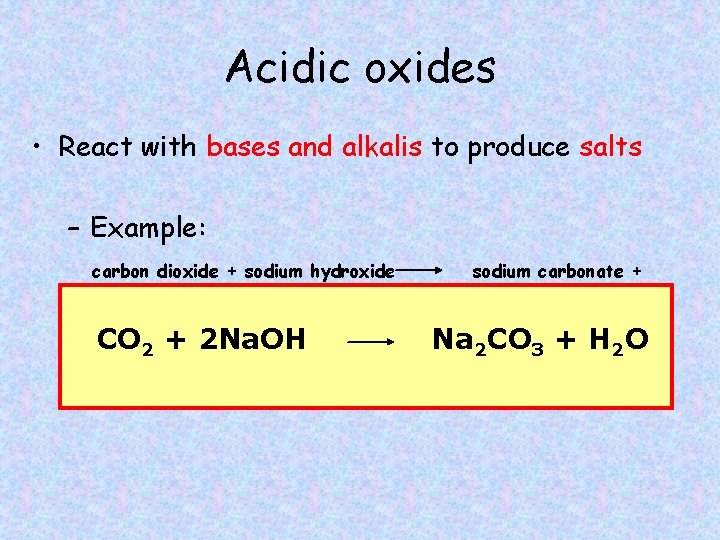
Acidic oxides • React with bases and alkalis to produce salts – Example: carbon dioxide + sodium hydroxide water CO 2 + 2 Na. OH sodium carbonate + Na 2 CO 3 + H 2 O
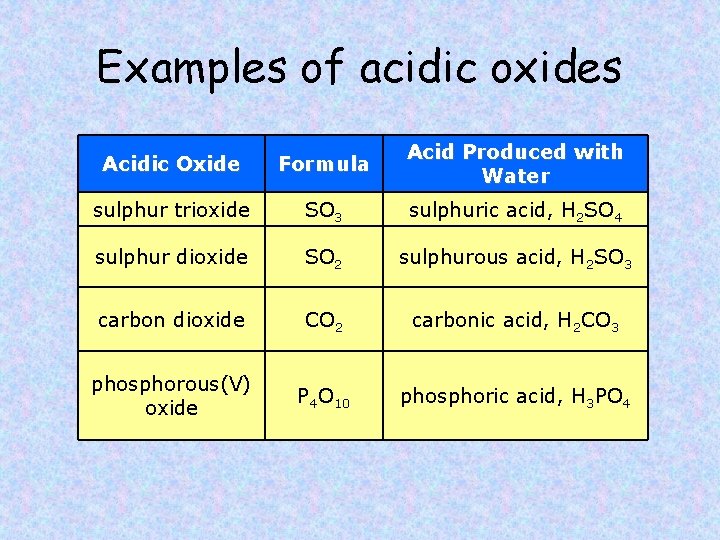
Examples of acidic oxides Acidic Oxide Formula Acid Produced with Water sulphur trioxide SO 3 sulphuric acid, H 2 SO 4 sulphur dioxide SO 2 sulphurous acid, H 2 SO 3 carbon dioxide CO 2 carbonic acid, H 2 CO 3 phosphorous(V) oxide P 4 O 10 phosphoric acid, H 3 PO 4

Basic oxides • Oxides of metal • Basic oxides are often solids at room temperature. • Most basic oxides are insoluble in water. Calcium oxide (quicklime)
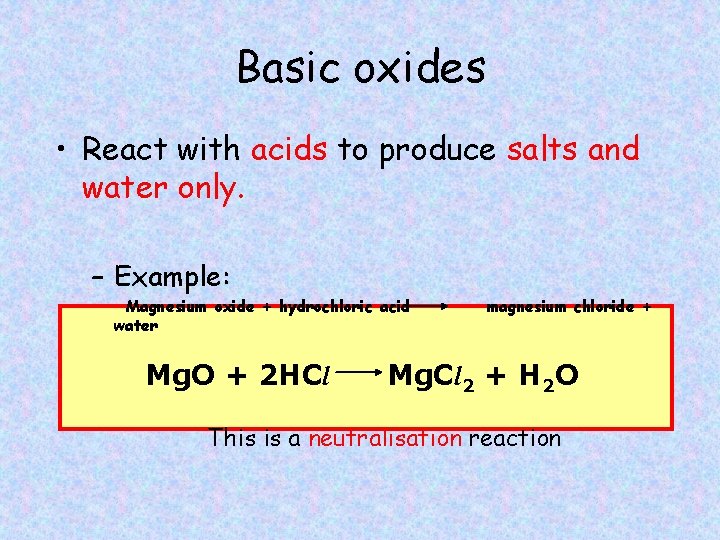
Basic oxides • React with acids to produce salts and water only. – Example: Magnesium oxide + hydrochloric acid water Mg. O + 2 HCl magnesium chloride + Mg. Cl 2 + H 2 O This is a neutralisation reaction
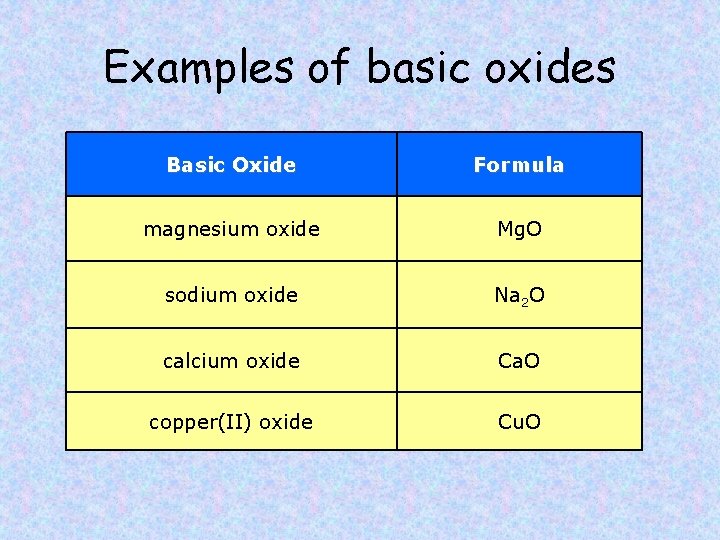
Examples of basic oxides Basic Oxide Formula magnesium oxide Mg. O sodium oxide Na 2 O calcium oxide Ca. O copper(II) oxide Cu. O

Amphoteric oxides • Oxides of metal • Can behave as acidic oxides or as basic oxides Zinc oxide

Neutral oxides • Non-metals that form oxides that show neither basic nor acidic properties. • Insoluble in water.
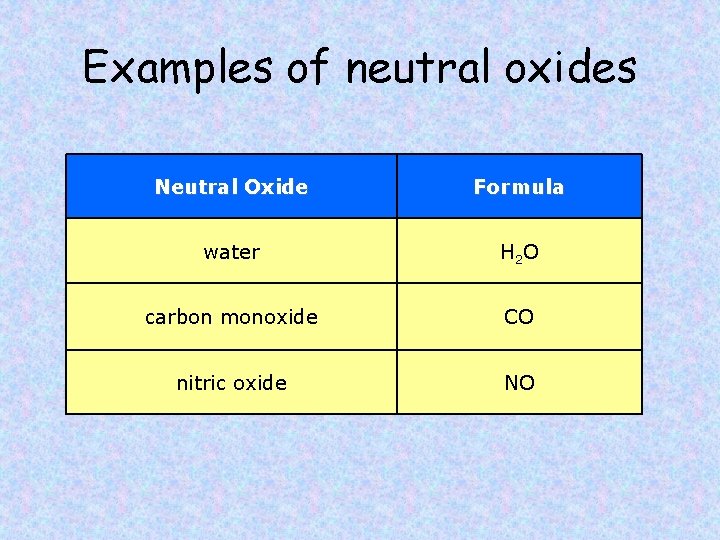
Examples of neutral oxides Neutral Oxide Formula water H 2 O carbon monoxide CO nitric oxide NO
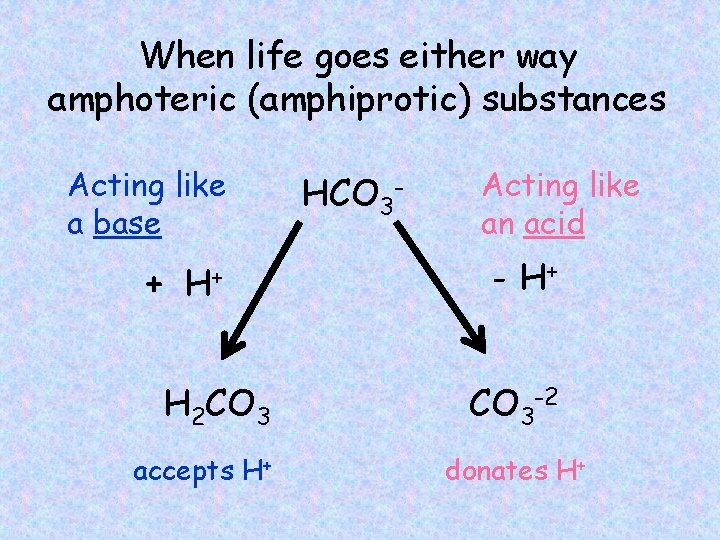
When life goes either way amphoteric (amphiprotic) substances Acting like a base + H+ H 2 CO 3 accepts H+ HCO 3 - Acting like an acid - H+ CO 3 -2 donates H+
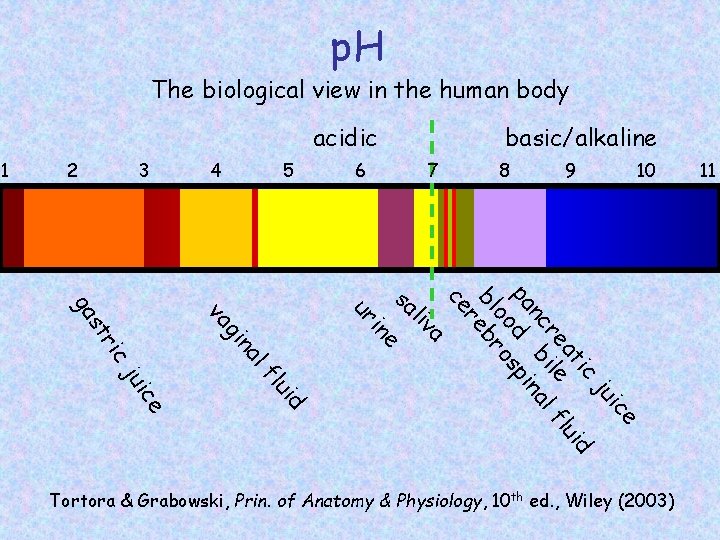
p. H The biological view in the human body acidic 1 2 3 4 5 basic/alkaline 6 7 8 9 10 e ic ju ic id at lu re ile l f nc b na pa d spi oo o bl br re ce liv sa ur a e in lu id ice ju lf ic na gi r st va ga Tortora & Grabowski, Prin. of Anatomy & Physiology, 10 th ed. , Wiley (2003) 11
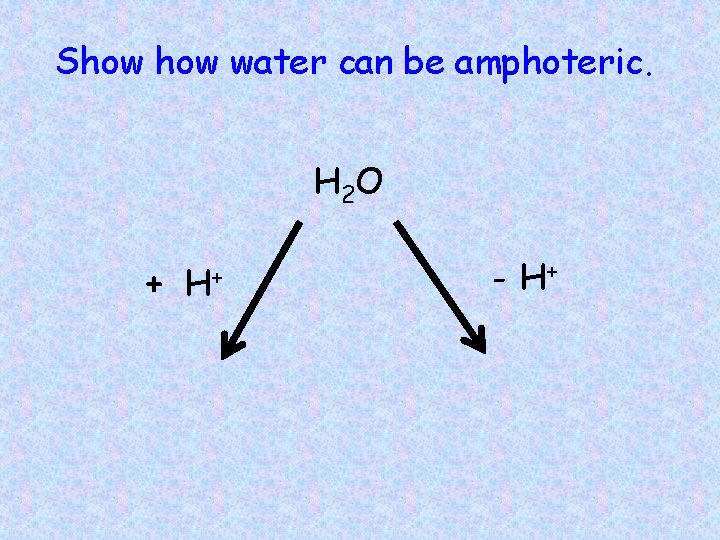
Show water can be amphoteric. H 2 O + H+ - H+
- Slides: 42