Acids and Bases Characteristics of Acids and Bases
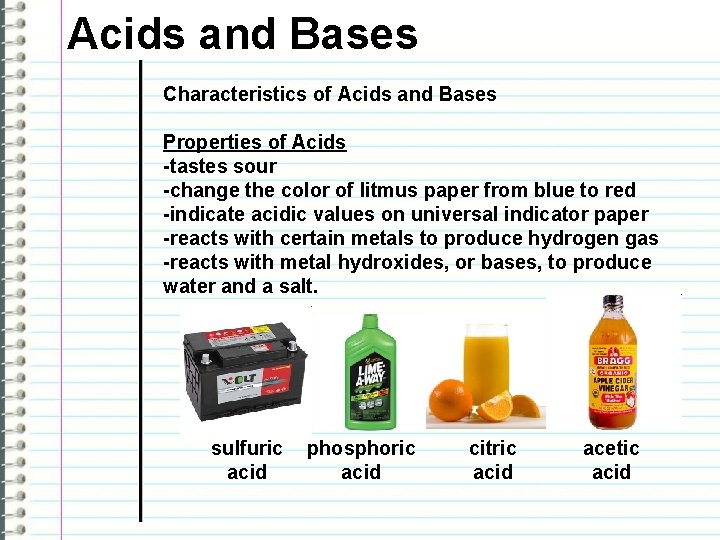
Acids and Bases Characteristics of Acids and Bases Properties of Acids -tastes sour -change the color of litmus paper from blue to red -indicate acidic values on universal indicator paper -reacts with certain metals to produce hydrogen gas -reacts with metal hydroxides, or bases, to produce water and a salt. sulfuric acid phosphoric acid citric acid acetic acid

Acids and Bases sodium hydroxide magnesium hydroxide Properties of Bases -tastes bitter or feels slippery -change the color of litmus paper from red to blue -indicate basic values on universal indicator paper -reacts with many compounds containing hydrogen ions, or acids, to produce water and a salt. ammonium hydroxide sodium bicarbonate

Acids and Bases To determine an acid or a base: -extracted pigment from red cabbage can be used as an indicator -basic solutions turn green, and acidic solutions turn red -universal indicator -litmus paper -p. H meter/probe

Acid or Base? If you tested an unknown substance, write down whether you think the following would be an acid or a based on each observation: 1. Upon putting the substance to your tongue, you find that it has a sour taste 2. After touching the substance, it feels slippery 3. When you taste the substance, it tastes bitter 4. Combining this substance with a known acid produces water and a salt 5. Putting a metal into a test tube of this substance yields hydrogen gas
- Slides: 4