Acids and Bases Chapter 32 What are Acids

Acids and Bases Chapter 32

What are Acids? • Acids are common chemicals, some of which are corrosive and dangerous and some of which are quiet harmless. Strong acids: Hydrochloric Acid(HCl) Sulfuric Acid(H 2 SO 4)
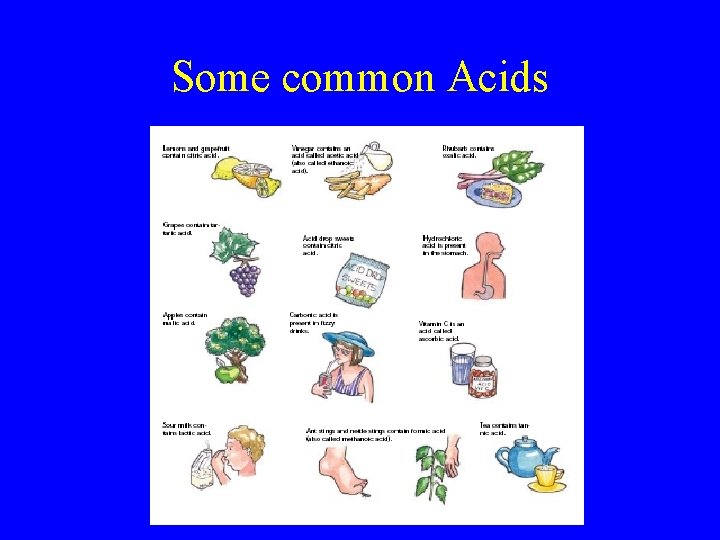
Some common Acids

Bases(or alkalis) • • Bases are opposite to acids Bases soluble in water are called alkalis. Alkalis have a soapy feel. Examples of bases: – Sodium Hydroxide Na. OH – Calcium Hydroxide Ca(OH)2

Indicators • An indicator shows by means of a colour change whether a substance is an acid or a base. • Acid turns Litmus from blue to red • Bases turn litmus from red to blue

The p. H scale • Litmus can only tell if a substance is an acid or base. It cannot tell the different strength of Acids or Bases. • The p. H scale indicates the level of acidity or basicity in a solution. – A solution that has a p. H of 7 is neutral – A solution that has a p. H less than 7 is acidic – A solution that has a p. H greater than 7 is alkaline.
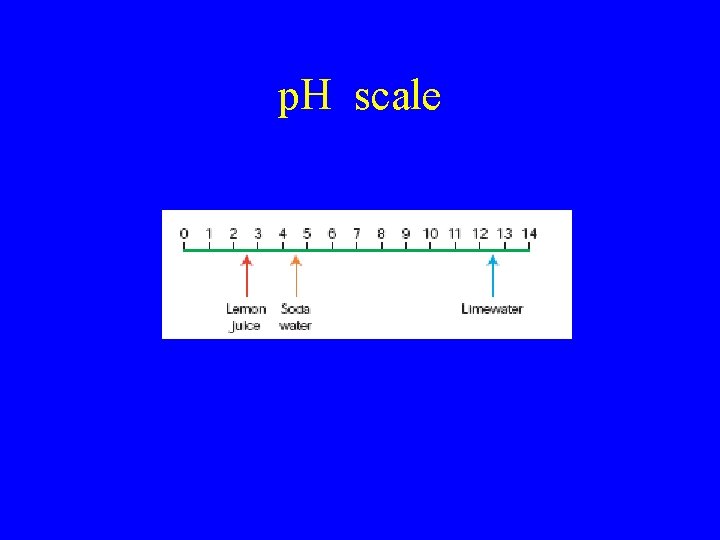
p. H scale

Universal Indicator • Universal Indicator can be used to find the exact p. H of a substance. • Universal indicator paper changes to a different colour depending on the p. H of the solution.

Reactions of Acids • Acids are involved in the following reactions. Acid + Base --> Salt + water Acid + Carbonate --> Salt + Water + Carbon Dioxide A salt is formed when the Hydrogen of an acid is replaced by a metal.
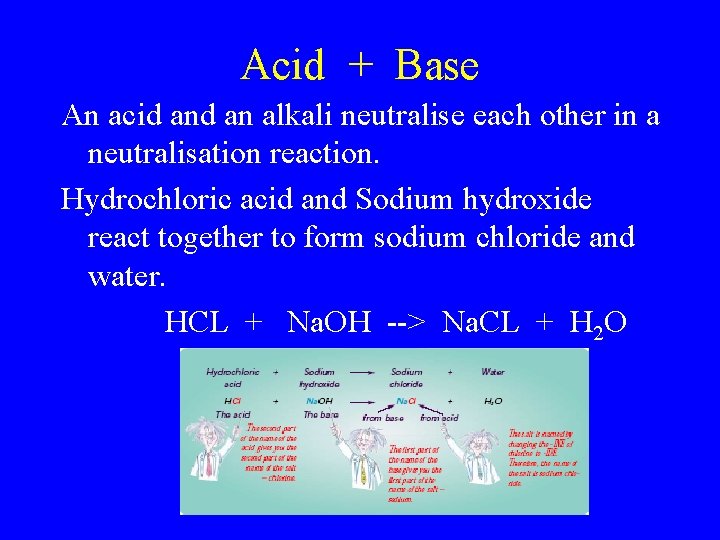
Acid + Base An acid an alkali neutralise each other in a neutralisation reaction. Hydrochloric acid and Sodium hydroxide react together to form sodium chloride and water. HCL + Na. OH --> Na. CL + H 2 O

To Neutralise an Acid and a Base by Titration Step 1. Using a pipette, place 20 ml of Sodium Hydroxide into a conical flask and a few drops of litmus indicator. Step 2. Fill the burette to the top mark with Hydrochloric acid. Step 3. Adjust level of meniscus of HCl in the burette to zero mark.

Step 4. Place the conical flask on a white tile Step 5. Start adding the acid to the base Step 6. The acid is added slowly until one drop turns the solution pink. When the indicator has changed colour, stop the titration and note the volume of acid added.

Step 7. Repeat the experiment but do not use indicator. Step 8. The sodium chloride is put in an evaporation dish. Step 9. The solution is evaporated to dryness and then left to cool Step 10. A white crystalline substance is formed and sodium chloride has been formed.
- Slides: 13