Acids and Bases Chapter 14 Properties of Acids

Acids and Bases Chapter 14

Properties of Acids: • taste sour (citrus fruits & vinegar) • affect indicators (e. g. turn blue litmus red) • produce H+ ions in aqueous solution • corrosive to metals • p. H < 7
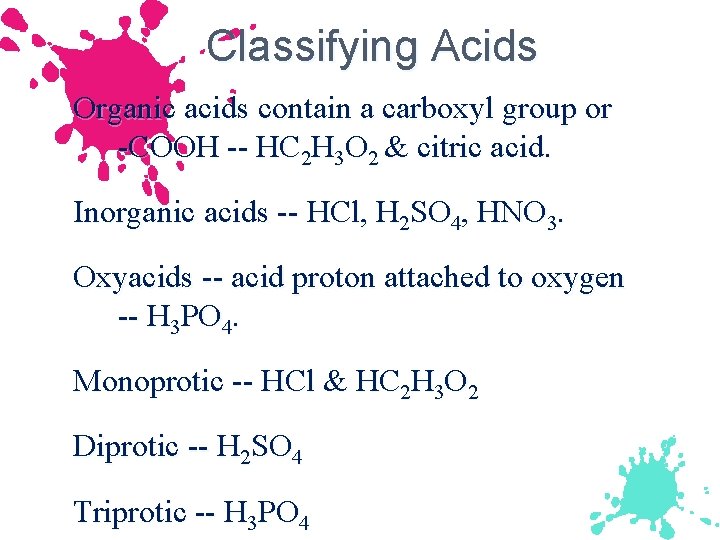
Classifying Acids Organic acids contain a carboxyl group or -COOH -- HC 2 H 3 O 2 & citric acid. Inorganic acids -- HCl, H 2 SO 4, HNO 3. Oxyacids -- acid proton attached to oxygen -- H 3 PO 4. Monoprotic -- HCl & HC 2 H 3 O 2 Diprotic -- H 2 SO 4 Triprotic -- H 3 PO 4

Properties of Bases: • taste bitter • feel slippery • affect indicators (e. g. turn red litmus blue) • produce OH- ions in aqueous solution • p. H > 7 • caustic
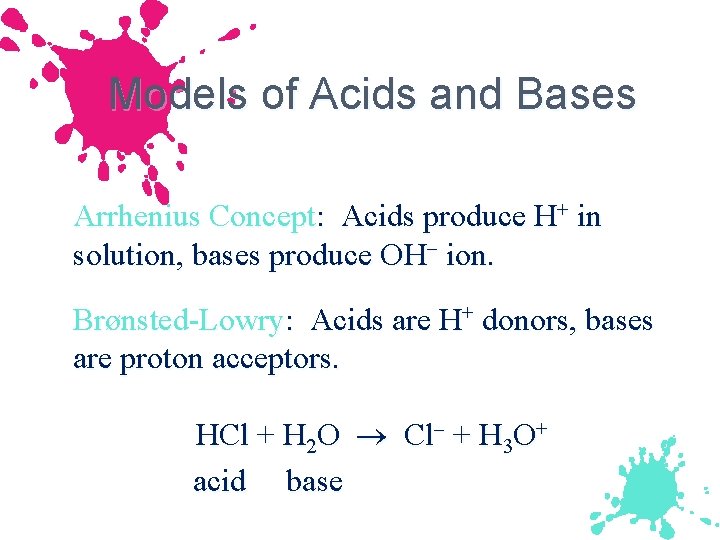
Models of Acids and Bases Arrhenius Concept: Acids produce H+ in solution, bases produce OH ion. Brønsted-Lowry: Acids are H+ donors, bases are proton acceptors. HCl + H 2 O Cl + H 3 O+ acid base
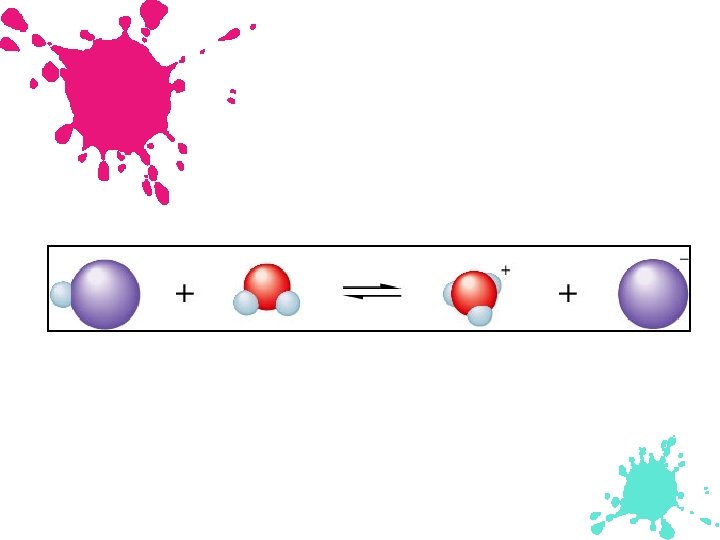

Hydronium Ion Hydronium ion is a hydrated proton -- . + H H The H+ ion is simply a proton. It has a very high charge density, so it strongly is attracted to the very electronegative oxygen of the polar water molecule. 2 O.
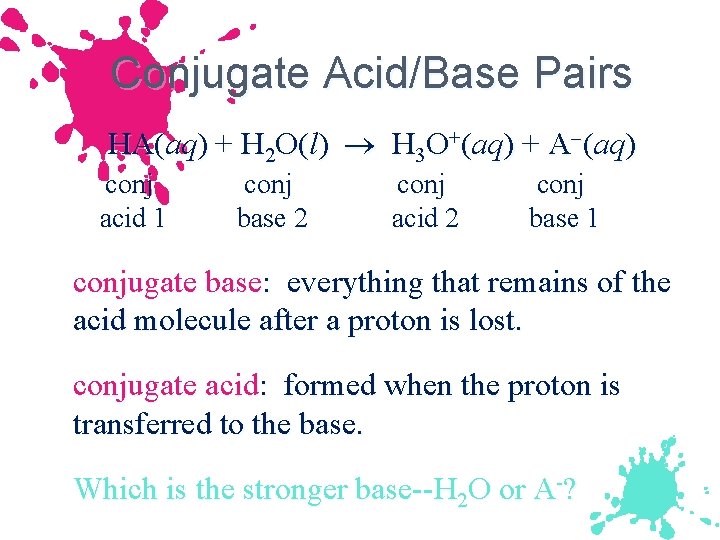
Conjugate Acid/Base Pairs HA(aq) + H 2 O(l) H 3 O+(aq) + A (aq) conj acid 1 conj base 2 conj acid 2 conj base 1 conjugate base: everything that remains of the acid molecule after a proton is lost. conjugate acid: formed when the proton is transferred to the base. Which is the stronger base--H 2 O or A-?
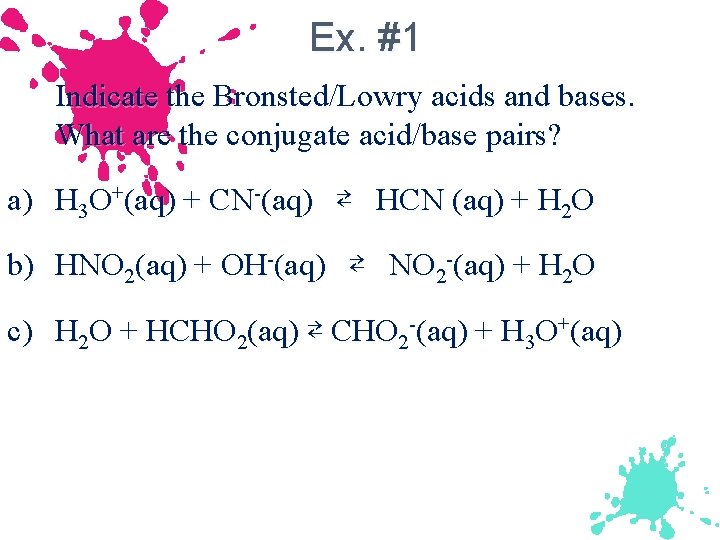
Ex. #1 Indicate the Bronsted/Lowry acids and bases. What are the conjugate acid/base pairs? a) H 3 O+(aq) + CN-(aq) ⇄ HCN (aq) + H 2 O b) HNO 2(aq) + OH-(aq) ⇄ NO 2 -(aq) + H 2 O c) H 2 O + HCHO 2(aq) ⇄ CHO 2 -(aq) + H 3 O+(aq)
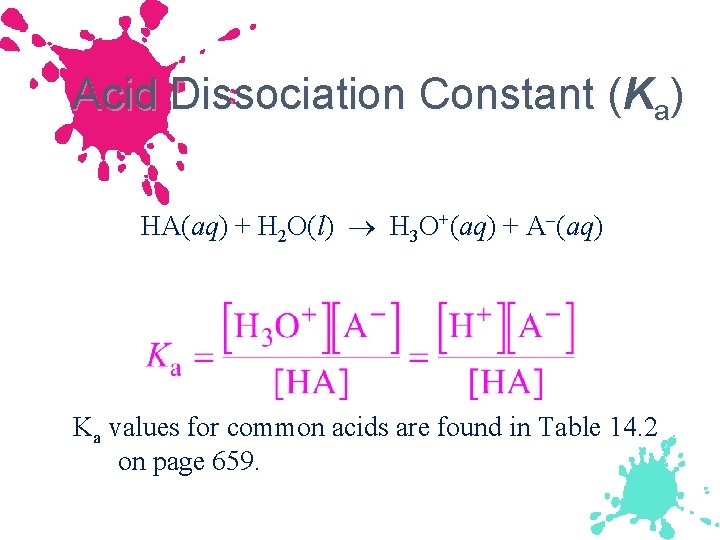
Acid Dissociation Constant (Ka) HA(aq) + H 2 O(l) H 3 O+(aq) + A (aq) Ka values for common acids are found in Table 14. 2 on page 659.

Acid Ionization Equilibrium

Example: Write the simple dissociation reaction for each of the following acids • HCl • HC 2 H 3 O 2 • NH 4+ • C 6 H 5 NH 3+ • [Al(H 2 O)6]3+

Example: Write the simple dissociation reaction for each of the following acids • HCl: HCl(aq)↔ H+(aq) + Cl(aq) • HC 2 H 3 O 2: HC 2 H 3 O 2(aq) ↔ H+(aq) + C 2 H 3 O 2 -(aq) • NH 4+: NH 4+(aq) ↔ H+(aq) + NH 3(aq) • C 6 H 5 NH 3+: C 6 H 5 NH 3+(aq) ↔ H+(aq) + C 5 H 5 NH 2(aq) • [Al(H 2 O)6]3+: Al(H 2 O)63+(aq)↔ H+(aq)+ Al(H 2 O)5(OH)2+(aq)

Bronsted-Lowry Model The Bronsted-Lowry Model is not limited to aqueous solutions like the Arrhenius Model. NH 3(g) + HCl(g) ----> NH 4 Cl(s) This is an acid-base reaction according to Bronsted-Lowry, but not according to Arrhenius!

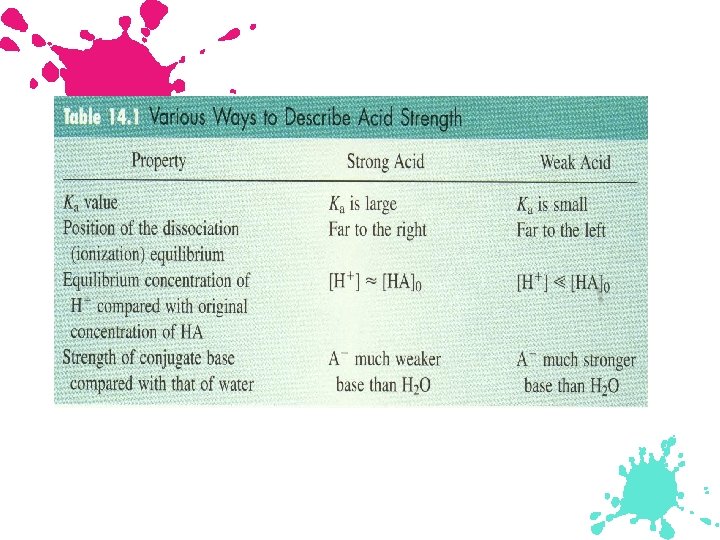
Acid Strength Strong Acid: - Its equilibrium position lies far to the right. (HNO 3) - Yields a weak conjugate base. (NO 3 )

Acid Strength (continued) Weak Acid: - Its equilibrium lies far to the left. (CH 3 COOH) - Yields a much stronger (water is relatively strong) conjugate base than water. (CH 3 COO )
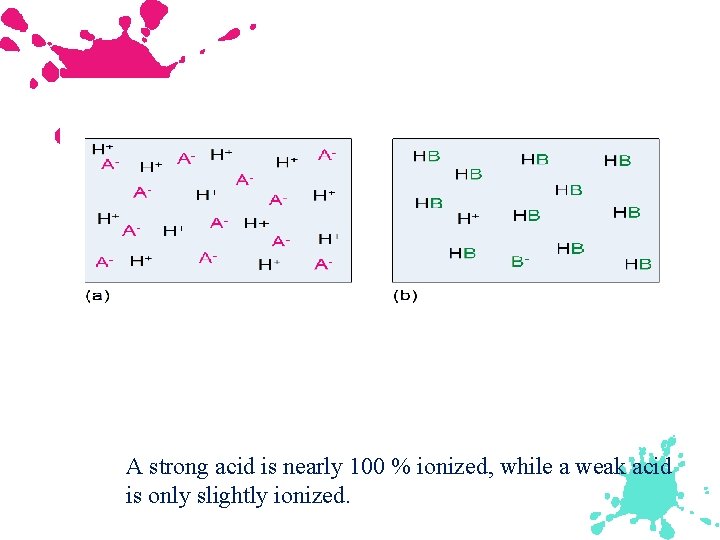
A strong acid is nearly 100 % ionized, while a weak acid is only slightly ionized.
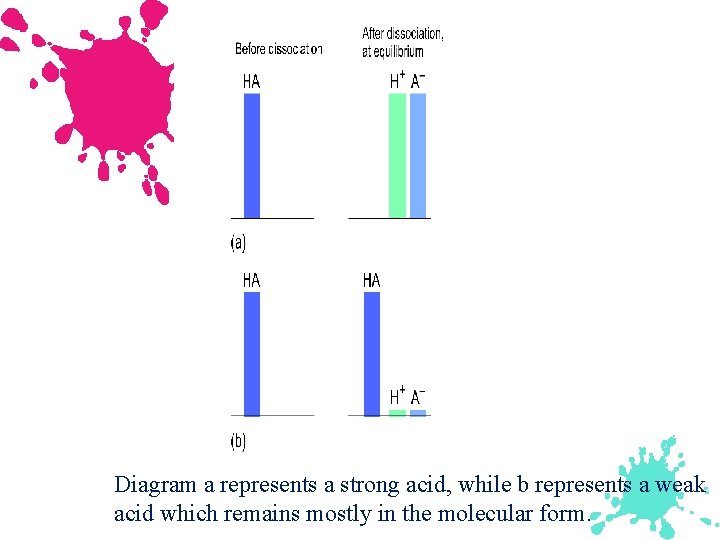
Diagram a represents a strong acid, while b represents a weak acid which remains mostly in the molecular form.
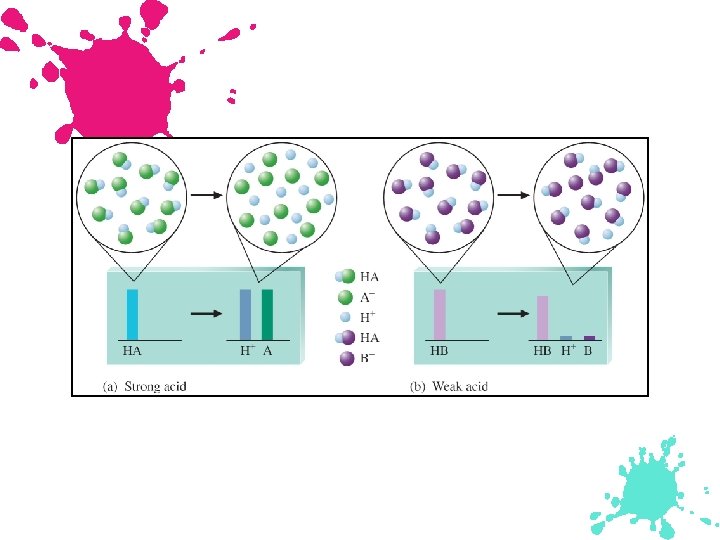
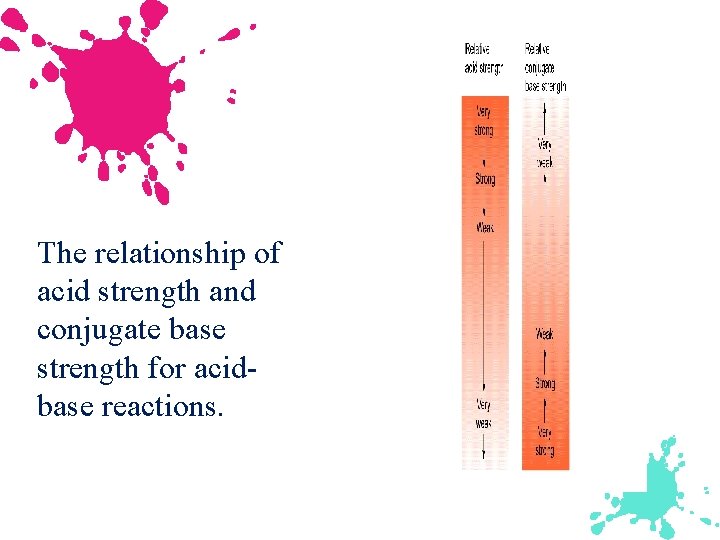
The relationship of acid strength and conjugate base strength for acidbase reactions.
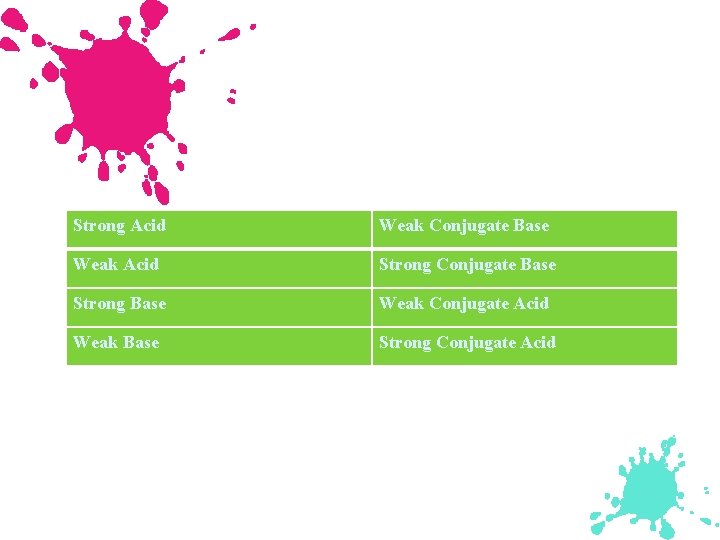
Strong Acid Weak Conjugate Base Weak Acid Strong Conjugate Base Strong Base Weak Conjugate Acid Weak Base Strong Conjugate Acid

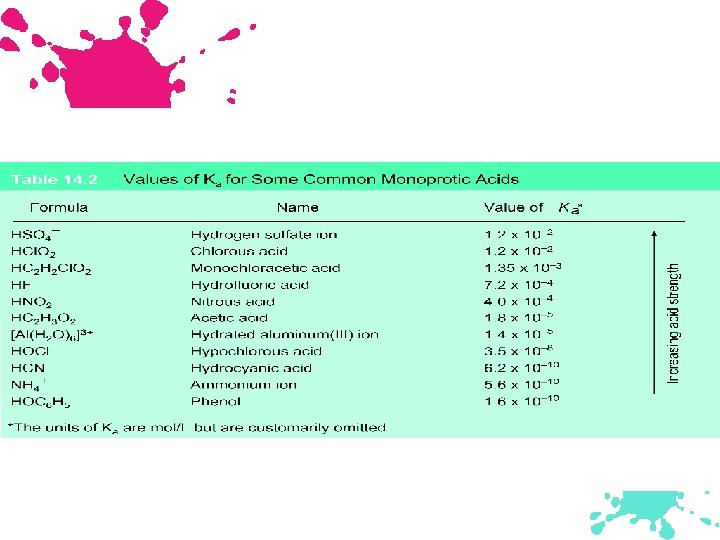
Arranging Species According to Increasing Basic Strength H 2 O, F-, Cl-, NO 2 -, & CNUse Table 14. 2 on page 659. Cl- is weakest since it is conjugate base of strong acid and weaker than water. Use Ka values to arrange the remaining bases. Cl- < H 2 O < F- < NO 2 - < CN-
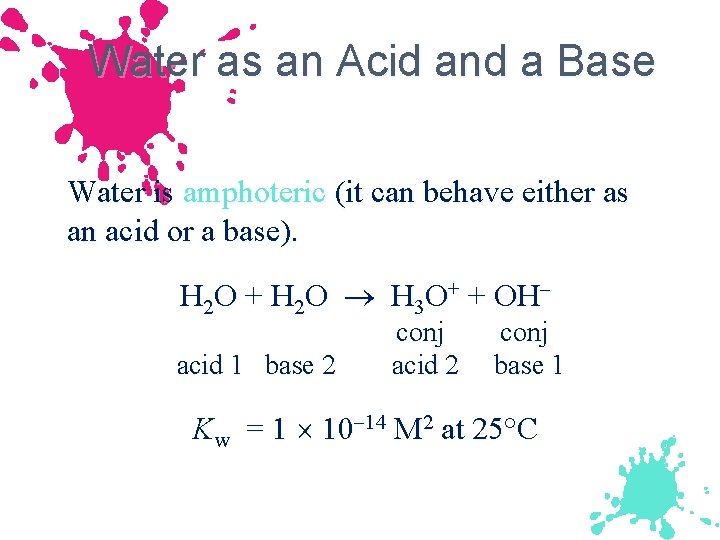
Water as an Acid and a Base Water is amphoteric (it can behave either as an acid or a base). H 2 O + H 2 O H 3 O+ + OH acid 1 base 2 conj acid 2 conj base 1 Kw = 1 10 14 M 2 at 25°C
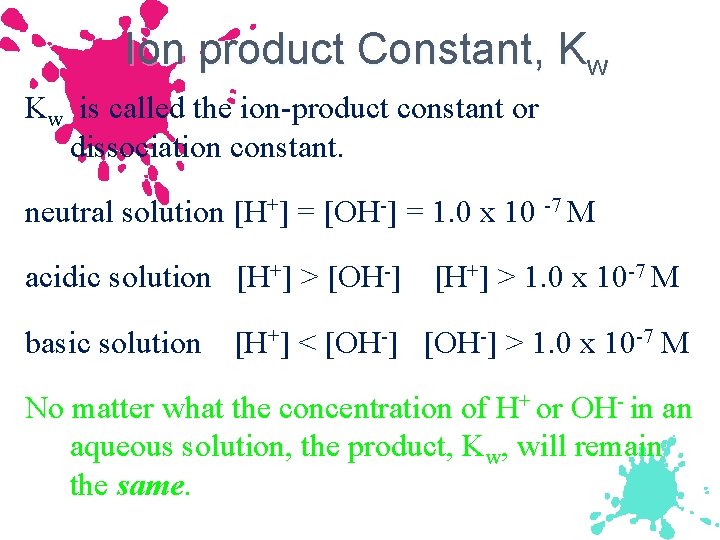
Ion product Constant, Kw Kw is called the ion-product constant or dissociation constant. neutral solution [H+] = [OH-] = 1. 0 x 10 -7 M acidic solution [H+] > [OH-] basic solution [H+] > 1. 0 x 10 -7 M [H+] < [OH-] > 1. 0 x 10 -7 M No matter what the concentration of H+ or OH- in an aqueous solution, the product, Kw, will remain the same.
![[H+] & [OH-] Calculations Calculate the [H+] for a 1. 0 x 10 -5 [H+] & [OH-] Calculations Calculate the [H+] for a 1. 0 x 10 -5](http://slidetodoc.com/presentation_image_h2/1f550dca989e533643fe96a246086379/image-27.jpg)
[H+] & [OH-] Calculations Calculate the [H+] for a 1. 0 x 10 -5 M OH-. Kw = [H+][OH-] [H+] = Kw/[OH-] [H+] = 1. 0 x 10 -14 M 2/1. 0 x 10 -5 M [H+] = 1. 0 x 10 -9 M
![[H+] & [OH-] Calculations Continued Calculate the [OH-] for a 10. 0 M H+. [H+] & [OH-] Calculations Continued Calculate the [OH-] for a 10. 0 M H+.](http://slidetodoc.com/presentation_image_h2/1f550dca989e533643fe96a246086379/image-28.jpg)
[H+] & [OH-] Calculations Continued Calculate the [OH-] for a 10. 0 M H+. Kw = [H+][OH-] = Kw/[H+] [OH-] = 1. 0 x 10 -14 M 2/10. 0 M [OH-] = 1. 0 x 10 -15 M
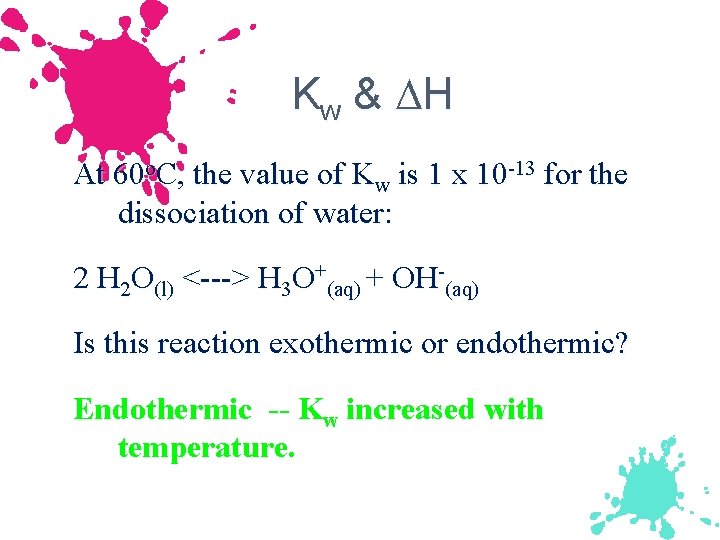
Kw & H At 60 o. C, the value of Kw is 1 x 10 -13 for the dissociation of water: 2 H 2 O(l) <---> H 3 O+(aq) + OH-(aq) Is this reaction exothermic or endothermic? Endothermic -- Kw increased with temperature.
![The p. H Scale p. H = log[H+] p. H in water usually ranges The p. H Scale p. H = log[H+] p. H in water usually ranges](http://slidetodoc.com/presentation_image_h2/1f550dca989e533643fe96a246086379/image-30.jpg)
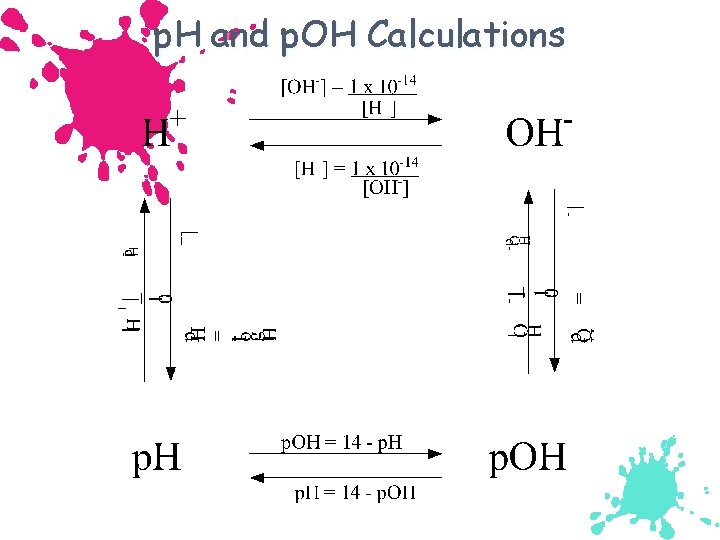
The p. H Scale p. H = log[H+] p. H in water usually ranges from 0 to 14. Kw = 1. 00 10 14 = [H+] [OH ] p. Kw = 14. 00 = p. H + p. OH As p. H rises, p. OH falls (sum = 14. 00).
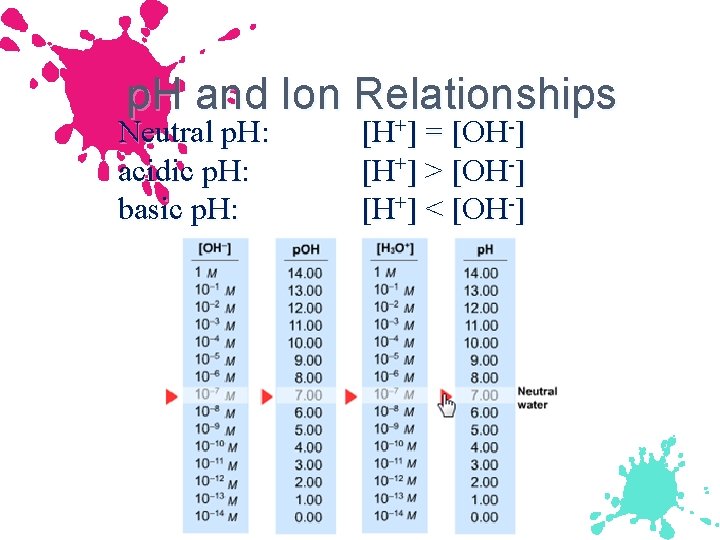
p. H and Ion Relationships + - Neutral p. H: acidic p. H: basic p. H: [H+] = [OH-] [H+] > [OH-] [H+] < [OH-]
![p. H & + [H ] p. H = 0 p. H = 7 p. H & + [H ] p. H = 0 p. H = 7](http://slidetodoc.com/presentation_image_h2/1f550dca989e533643fe96a246086379/image-32.jpg)
p. H & + [H ] p. H = 0 p. H = 7 p. H = 14 1 x 10 -14 1 x 10 -7 1 x 100 OH - H 3 + O OHH 3 O+ OH H 3 O + 1 x 100 1 x 10 -7 1 x 10 -14
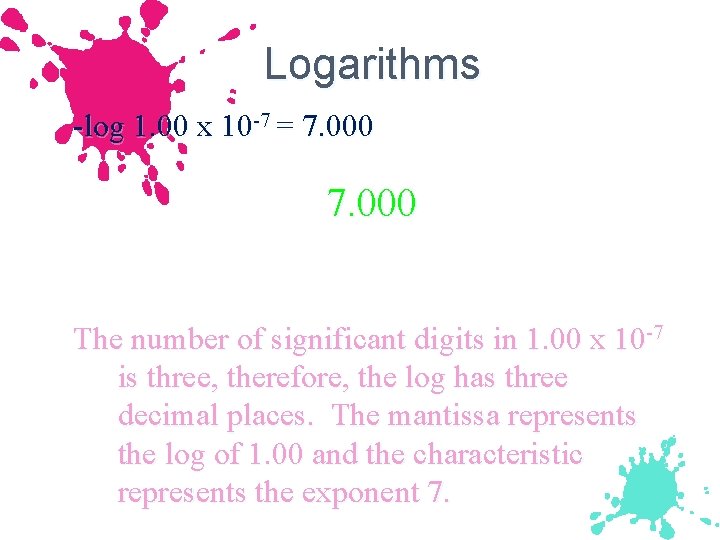
Logarithms -log 1. 00 x 10 -7 = 7. 000 The number of significant digits in 1. 00 x 10 -7 is three, therefore, the log has three decimal places. The mantissa represents the log of 1. 00 and the characteristic represents the exponent 7.

p. H scale and p. H values for common substances. A p. H of 1 is 100 times more acidic than a p. H of 3.
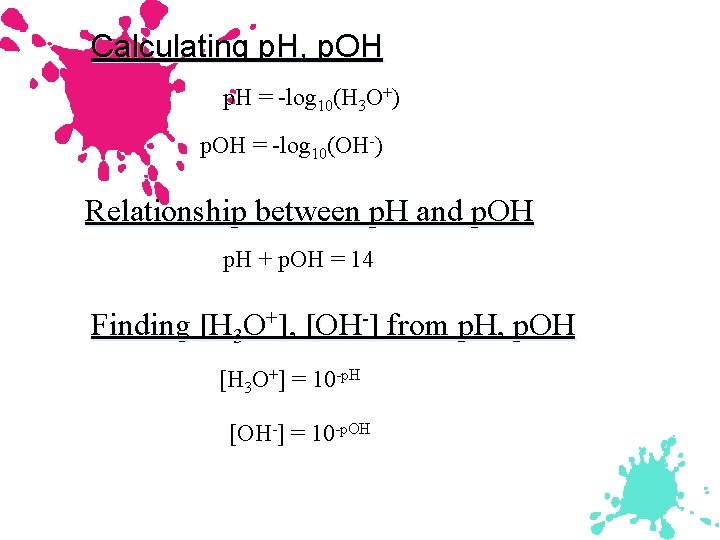
Calculating p. H, p. OH p. H = -log 10(H 3 O+) p. OH = -log 10(OH-) Relationship between p. H and p. OH p. H + p. OH = 14 Finding [H 3 O+], [OH-] from p. H, p. OH [H 3 O+] = 10 -p. H [OH-] = 10 -p. OH

p. H and p. OH Calculations
![p. H Calculations What is the p. OH, [H+], & [OH-] for human blood p. H Calculations What is the p. OH, [H+], & [OH-] for human blood](http://slidetodoc.com/presentation_image_h2/1f550dca989e533643fe96a246086379/image-37.jpg)
p. H Calculations What is the p. OH, [H+], & [OH-] for human blood with a p. H of 7. 41? p. H + p. OH = 14. 00 - p. H p. OH = 14. 00 - 7. 41 p. OH = 6. 59
![p. H Calculations Continued What is the p. OH, [H+], & [OH-] for human p. H Calculations Continued What is the p. OH, [H+], & [OH-] for human](http://slidetodoc.com/presentation_image_h2/1f550dca989e533643fe96a246086379/image-38.jpg)
p. H Calculations Continued What is the p. OH, [H+], & [OH-] for human blood with a p. H of 7. 41? p. H = - log [H+] = antilog (-p. H) [H+] = antilog (-7. 41) [H+] = 3. 9 x 10 -8 M Note: The number of significant figures in the antilog is equal to the number of decimal places in the p. H.
![p. H Calculations Continued What is the p. OH, [H+], & [OH-] for human p. H Calculations Continued What is the p. OH, [H+], & [OH-] for human](http://slidetodoc.com/presentation_image_h2/1f550dca989e533643fe96a246086379/image-39.jpg)
p. H Calculations Continued What is the p. OH, [H+], & [OH-] for human blood with a p. H of 7. 41? p. OH = - log [OH-] = antilog (-p. OH) [OH-] = antilog (-6. 59) [OH-] = 2. 6 x 10 -7 M Note: The number of significant figures in the antilog is equal to the number of decimal places in the p. OH.

Thinking About Acid–Base Problems What are the major species in solution? What is the dominant reaction that will take place? § Is it an equilibrium reaction or a reaction that will go essentially to completion? § React all major species until you are left with an equilibrium reaction. Solve for the p. H if needed.
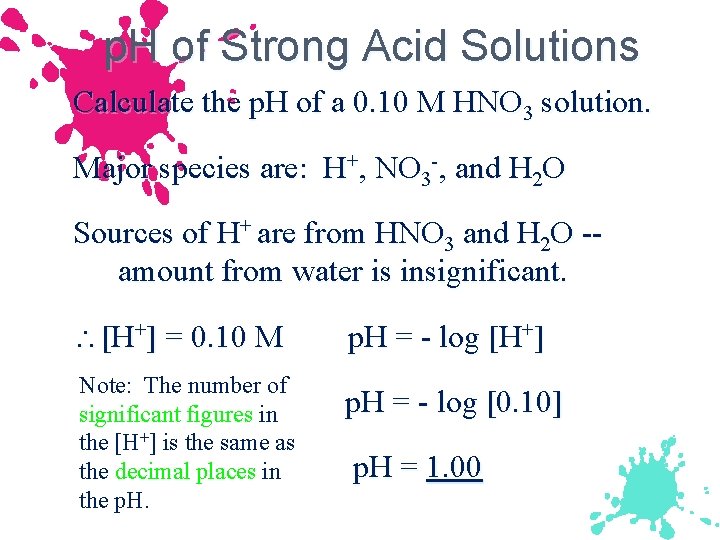
p. H of Strong Acid Solutions Calculate the p. H of a 0. 10 M HNO 3 solution. Major species are: H+, NO 3 -, and H 2 O Sources of H+ are from HNO 3 and H 2 O -amount from water is insignificant. [H+] = 0. 10 M Note: The number of significant figures in the [H+] is the same as the decimal places in the p. H = - log [H+] p. H = - log [0. 10] p. H = 1. 00
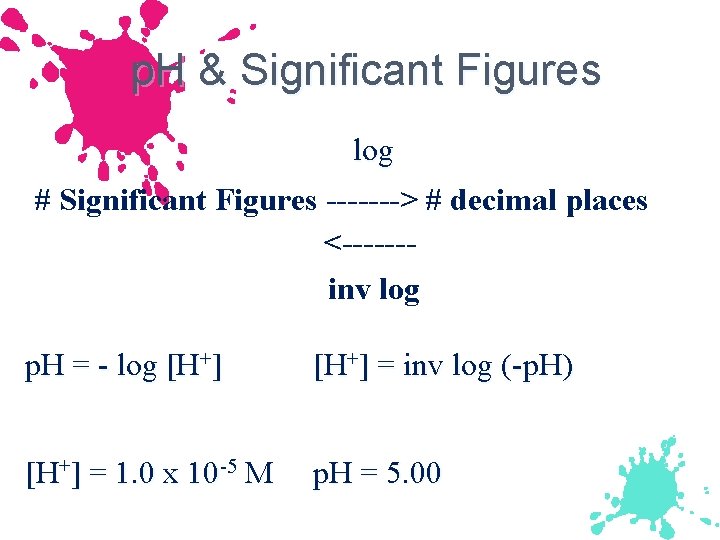
p. H & Significant Figures log # Significant Figures -------> # decimal places <------inv log p. H = - log [H+] = inv log (-p. H) [H+] = 1. 0 x 10 -5 M p. H = 5. 00

Solving Weak Acid Equilibrium Problems - List major species in solution. - Choose species that can produce H+ and write reactions. - Based on K values, decide on dominant equilibrium. - Write equilibrium expression for dominant equilibrium. - List initial concentrations in dominant equilibrium.

Solving Weak Acid Equilibrium Problems (continued) - Define change at equilibrium (as “x”). - Write equilibrium concentrations in terms of x. - Substitute equilibrium concentrations into equilibrium expression. - Solve for x the “easy way. ” x can be neglected when concentration is 2 powers of 10 (100 x) greater than Ka or Kb. - Verify assumptions using 5% rule. - Calculate [H+] and p. H.

p. H of Weak Acid Solutions Calculate the p. H of a 0. 100 M HOCl solution. Major species: HOCl and HOH Ka HOCl = 3. 5 x 10 -8 & Ka HOH = 1. 0 x 10 -14 HOCl will be only significant source of [H+]. Ka = 3. 5 x 10 -8 = [H+][OCl-]/[HOCl]
![p. H of Weak Acid Solutions Continued ICE [HOCl] [OCl-] [H+] Initial (mol/L) 0. p. H of Weak Acid Solutions Continued ICE [HOCl] [OCl-] [H+] Initial (mol/L) 0.](http://slidetodoc.com/presentation_image_h2/1f550dca989e533643fe96a246086379/image-46.jpg)
p. H of Weak Acid Solutions Continued ICE [HOCl] [OCl-] [H+] Initial (mol/L) 0. 100 0 0 Change (mol/L) -x +x +x 0+x Equil. (mol/L) 0. 100 - x
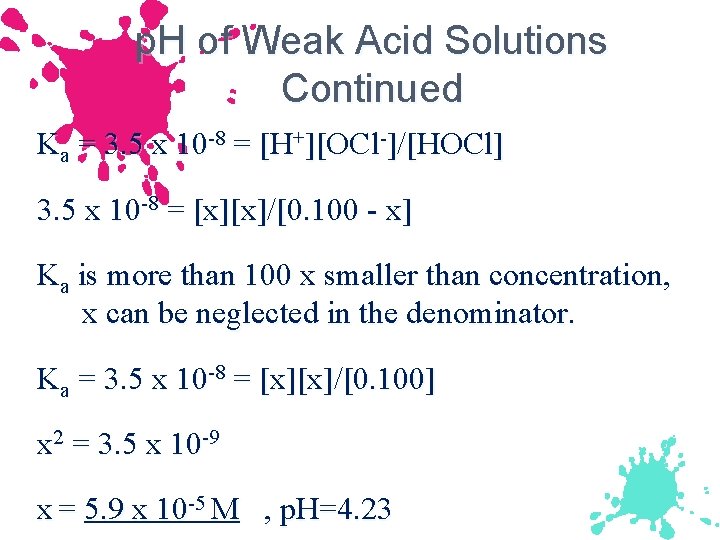
p. H of Weak Acid Solutions Continued Ka = 3. 5 x 10 -8 = [H+][OCl-]/[HOCl] 3. 5 x 10 -8 = [x][x]/[0. 100 - x] Ka is more than 100 x smaller than concentration, x can be neglected in the denominator. Ka = 3. 5 x 10 -8 = [x][x]/[0. 100] x 2 = 3. 5 x 10 -9 x = 5. 9 x 10 -5 M , p. H=4. 23
![p. H of Weak Acid Solutions Continued Approximation check: % dissociation = (x/[HA]o) (100%) p. H of Weak Acid Solutions Continued Approximation check: % dissociation = (x/[HA]o) (100%)](http://slidetodoc.com/presentation_image_h2/1f550dca989e533643fe96a246086379/image-48.jpg)
p. H of Weak Acid Solutions Continued Approximation check: % dissociation = (x/[HA]o) (100%) % dissociation = (x/[HOCl]o) (100%) % dissociation = (5. 9 x 10 -5/0. 100)(100%) % dissociation = 0. 059 % This is much less than 5 % and therefore the approximation was valid.

p. H of a mixture of weak acids • Calculate the p. H of a solution that contains 1. 00 M HCN (Ka=6. 2 x 10 -10), 5. 00 M HNO 2 (Ka=4. 0 x 10 -4). Also calculate the concentration of CN- in this solution at equilibrium. Ø Since HCN & HNO 2 are weak acids and are largely undissociated, the major species are: H 2 O, HCN, HNO 2 Ø ONLY CARE ABOUT HNO 2 because Ka is MUCH larger than HCN & H 2 O

You can walk through the first part of the problem on page 671. Answers: [H+]=4. 5 x 10 -2 M, p. H=1. 35 We’re going to walk through calculating CNconcentration

HCN(aq) ↔ H+(aq) + CN-(aq) - We already know Ka is 6. 2 x 10 -10 - Set up Ka=[H+][CN] / [HCN] - Get [H+] concentration from first part of problem
![- We know that [HCN]o=1. 00 M, and since Ka for HCN is so - We know that [HCN]o=1. 00 M, and since Ka for HCN is so](http://slidetodoc.com/presentation_image_h2/1f550dca989e533643fe96a246086379/image-52.jpg)
- We know that [HCN]o=1. 00 M, and since Ka for HCN is so small, a negligible amount will dissociate - Thus, [HCN]≈[HCN]o = 1. 00 - 6. 2 x 10 -10 = [4. 5 x 10 -2][CN] [1. 00] - [CN]= [(6. 2 x 10 -10) (1. 00)] / (4. 5 x 10 -2) - [CN] = 1. 4 x 10 -8 M
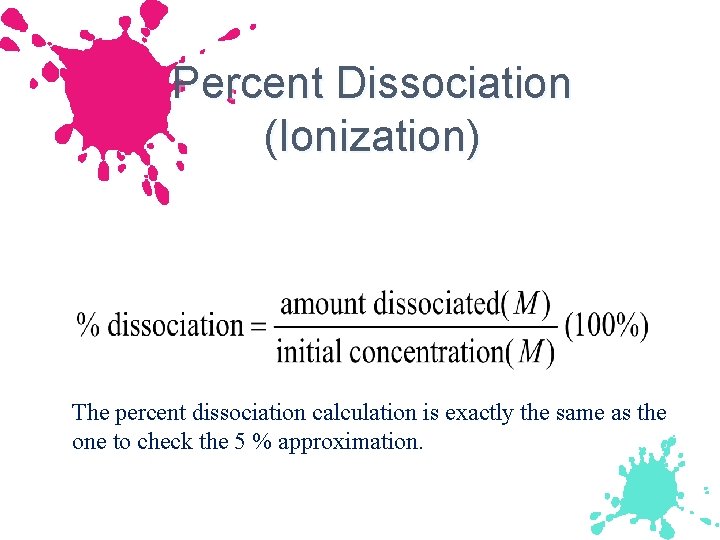
Percent Dissociation (Ionization) The percent dissociation calculation is exactly the same as the one to check the 5 % approximation.
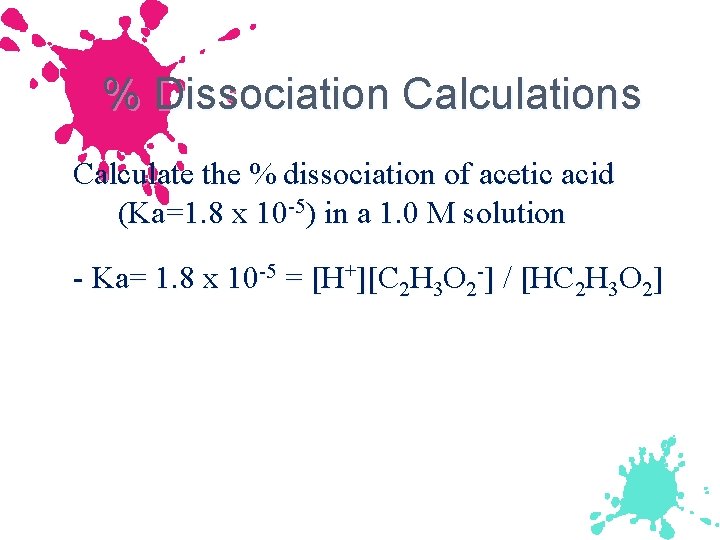
% Dissociation Calculations Calculate the % dissociation of acetic acid (Ka=1. 8 x 10 -5) in a 1. 0 M solution - Ka= 1. 8 x 10 -5 = [H+][C 2 H 3 O 2 -] / [HC 2 H 3 O 2]
![% Dissociation Calcs ICE [HC 2 H 3 O 2] [H+] Initial (mol/L) 1. % Dissociation Calcs ICE [HC 2 H 3 O 2] [H+] Initial (mol/L) 1.](http://slidetodoc.com/presentation_image_h2/1f550dca989e533643fe96a246086379/image-55.jpg)
% Dissociation Calcs ICE [HC 2 H 3 O 2] [H+] Initial (mol/L) 1. 00 0 0 Change (mol/L) -x +x +x 1. 00 - x 0+x Equil. (mol/L) [C 2 H 3 O 2 -]
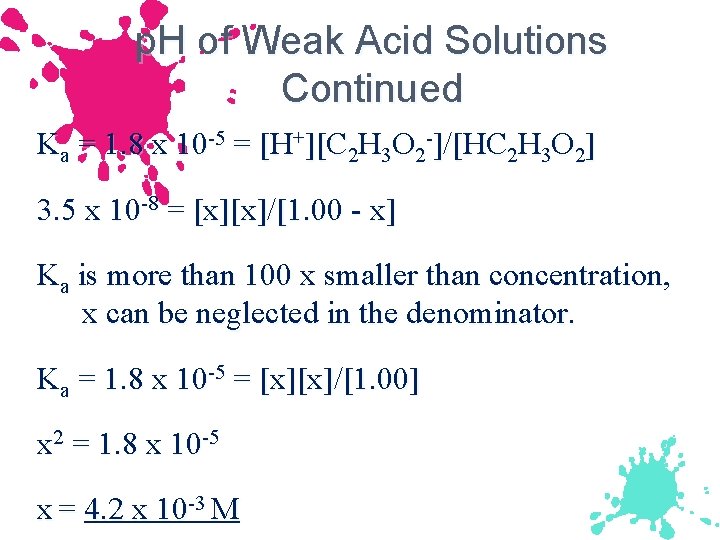
p. H of Weak Acid Solutions Continued Ka = 1. 8 x 10 -5 = [H+][C 2 H 3 O 2 -]/[HC 2 H 3 O 2] 3. 5 x 10 -8 = [x][x]/[1. 00 - x] Ka is more than 100 x smaller than concentration, x can be neglected in the denominator. Ka = 1. 8 x 10 -5 = [x][x]/[1. 00] x 2 = 1. 8 x 10 -5 x = 4. 2 x 10 -3 M
![% Dissociation Calcs Percent Dissociation = ([H+]/[HC 2 H 3 O 2]o) x 100 % Dissociation Calcs Percent Dissociation = ([H+]/[HC 2 H 3 O 2]o) x 100](http://slidetodoc.com/presentation_image_h2/1f550dca989e533643fe96a246086379/image-57.jpg)
% Dissociation Calcs Percent Dissociation = ([H+]/[HC 2 H 3 O 2]o) x 100 =(4. 2 x 10 -3) / (1. 00) =. 0042 x 100 = 0. 42% diss. • For solutions of any weak acid, HA, [H+] decreases as [HA]o decreases BUT % dissociation increases and [HA] decreases

Or stated as… • For a given weak acid, the percent dissociation increases as the acid becomes more dilute.
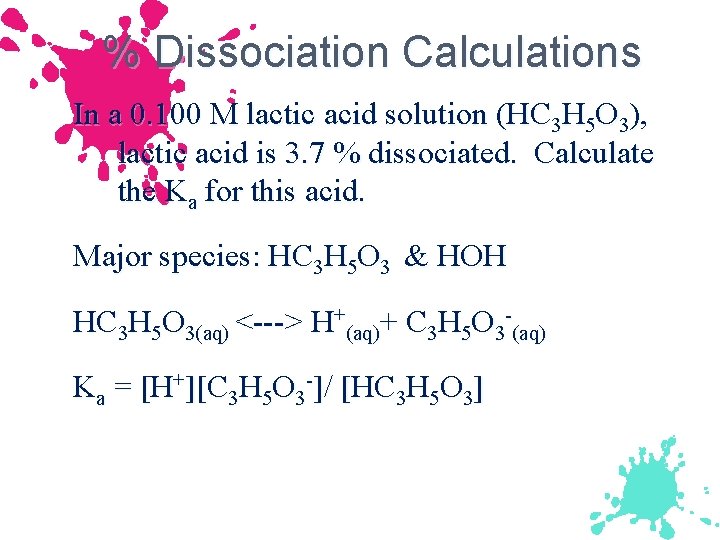
% Dissociation Calculations In a 0. 100 M lactic acid solution (HC 3 H 5 O 3), lactic acid is 3. 7 % dissociated. Calculate the Ka for this acid. Major species: HC 3 H 5 O 3 & HOH HC 3 H 5 O 3(aq) <---> H+(aq)+ C 3 H 5 O 3 -(aq) Ka = [H+][C 3 H 5 O 3 -]/ [HC 3 H 5 O 3]
![% Dissociation Calculations Continued ICE [HC 3 H 5 O 3] Initial (M) 0. % Dissociation Calculations Continued ICE [HC 3 H 5 O 3] Initial (M) 0.](http://slidetodoc.com/presentation_image_h2/1f550dca989e533643fe96a246086379/image-60.jpg)
% Dissociation Calculations Continued ICE [HC 3 H 5 O 3] Initial (M) 0. 10 [C 3 H 5 O 3 -] 0 [H+] 0 Change (M) - 3. 7 x 10 -3 + 3. 7 x 10 -3 Equil. (M) 0. 10 + 3. 7 x 10 -3
![% Dissociation Calculations Continued Ka = [H+][C 3 H 5 O 3 -]/ [HC % Dissociation Calculations Continued Ka = [H+][C 3 H 5 O 3 -]/ [HC](http://slidetodoc.com/presentation_image_h2/1f550dca989e533643fe96a246086379/image-61.jpg)
% Dissociation Calculations Continued Ka = [H+][C 3 H 5 O 3 -]/ [HC 3 H 5 O 3] Ka = [3. 7 x 10 -3]2/ [0. 10] Ka = 1. 4 x 10 -4
![The effect of dilution on the % dissociation and [H+] of a weak acid The effect of dilution on the % dissociation and [H+] of a weak acid](http://slidetodoc.com/presentation_image_h2/1f550dca989e533643fe96a246086379/image-62.jpg)
The effect of dilution on the % dissociation and [H+] of a weak acid solution.

Bases are often called alkalis because they often contain alkali or alkaline earth metals. “Strong” and “weak” are used in the same sense for bases as for acids. strong = complete dissociation (hydroxide ion supplied to solution) Na. OH(s) Na+(aq) + OH (aq)
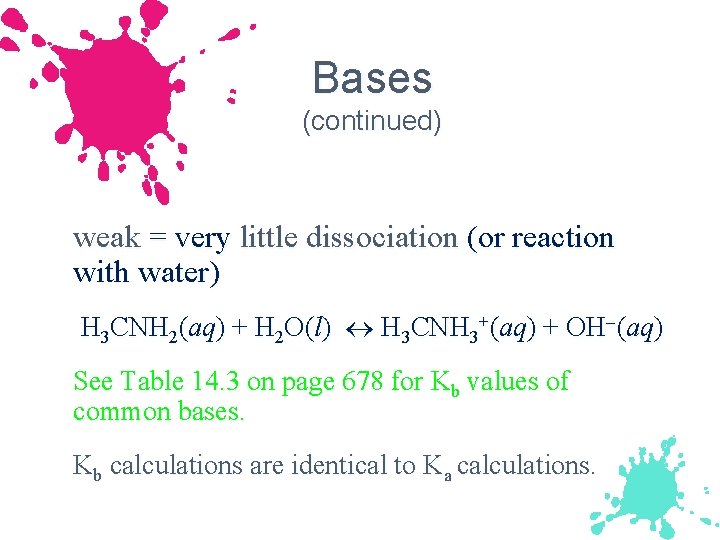
Bases (continued) weak = very little dissociation (or reaction with water) H 3 CNH 2(aq) + H 2 O(l) H 3 CNH 3+(aq) + OH (aq) See Table 14. 3 on page 678 for Kb values of common bases. Kb calculations are identical to Ka calculations.
p. H and p. OH Calculations
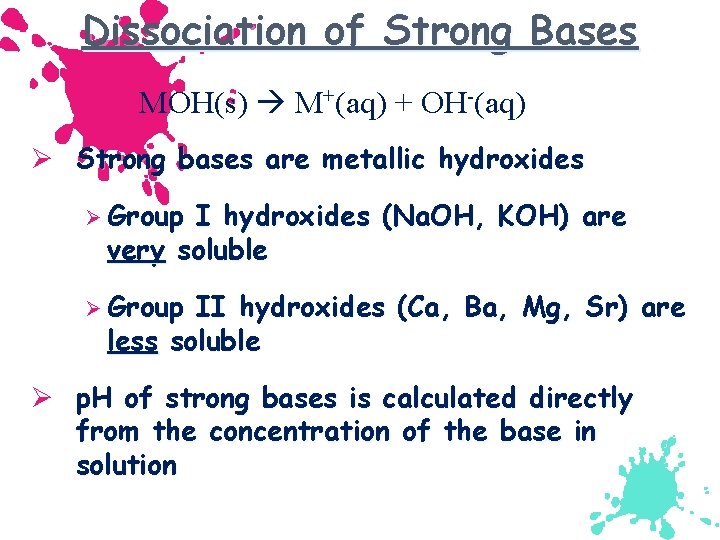
Dissociation of Strong Bases MOH(s) M+(aq) + OH-(aq) Ø Strong bases are metallic hydroxides Ø Group I hydroxides (Na. OH, KOH) are very soluble Ø Group II hydroxides (Ca, Ba, Mg, Sr) are less soluble Ø p. H of strong bases is calculated directly from the concentration of the base in solution
![Calculate the following [OH-], p. OH, and [H+] for the following STRONG bases 1. Calculate the following [OH-], p. OH, and [H+] for the following STRONG bases 1.](http://slidetodoc.com/presentation_image_h2/1f550dca989e533643fe96a246086379/image-67.jpg)
Calculate the following [OH-], p. OH, and [H+] for the following STRONG bases 1. 1. 0 x 10– 3 M solution of sodium hydroxide 2. 1. 0 x 10– 3 M solution of calcium hydroxide
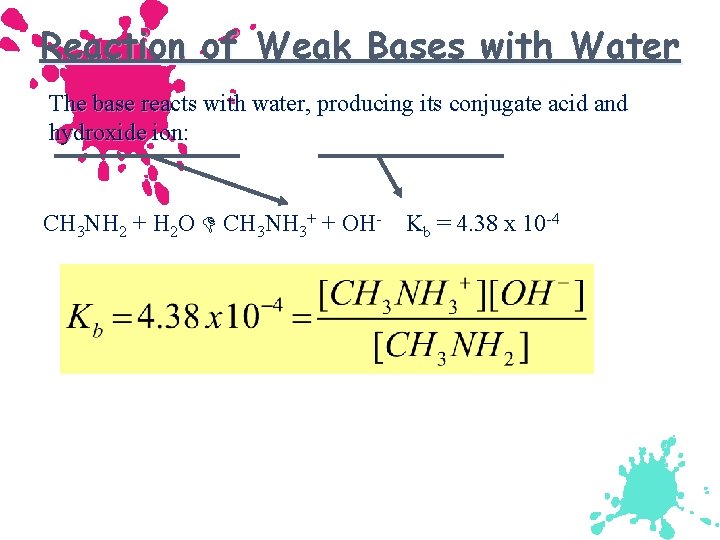
Reaction of Weak Bases with Water The base reacts with water, producing its conjugate acid and hydroxide ion: CH 3 NH 2 + H 2 O CH 3 NH 3+ + OH- Kb = 4. 38 x 10 -4
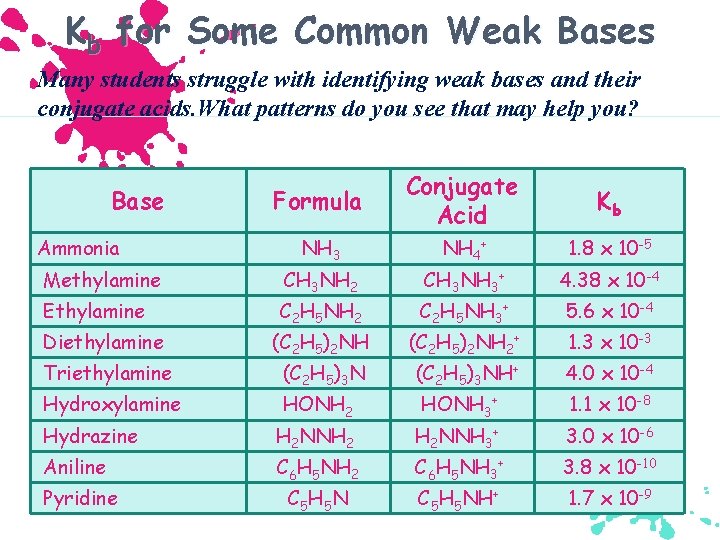
Kb for Some Common Weak Bases Many students struggle with identifying weak bases and their conjugate acids. What patterns do you see that may help you? Formula Conjugate Acid Kb NH 3 NH 4+ 1. 8 x 10 -5 Methylamine CH 3 NH 2 CH 3 NH 3+ 4. 38 x 10 -4 Ethylamine C 2 H 5 NH 2 C 2 H 5 NH 3+ 5. 6 x 10 -4 Diethylamine (C 2 H 5)2 NH 2+ 1. 3 x 10 -3 Triethylamine (C 2 H 5)3 NH+ 4. 0 x 10 -4 Hydroxylamine HONH 2 HONH 3+ 1. 1 x 10 -8 Base Ammonia Hydrazine H 2 NNH 2 H 2 NNH 3+ 3. 0 x 10 -6 Aniline C 6 H 5 NH 2 C 6 H 5 NH 3+ 3. 8 x 10 -10 Pyridine C 5 H 5 NH+ 1. 7 x 10 -9
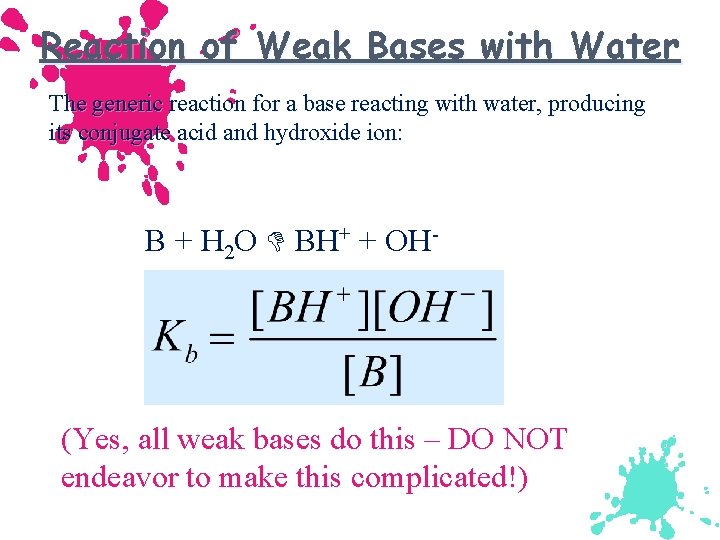
Reaction of Weak Bases with Water The generic reaction for a base reacting with water, producing its conjugate acid and hydroxide ion: B + H 2 O BH+ + OH- (Yes, all weak bases do this – DO NOT endeavor to make this complicated!)

A Weak Base Equilibrium Problem What is the p. H of a 0. 50 M solution of ammonia, NH 3, Kb = 1. 8 x 10 -5 ? Step #1: Write the equation for the reaction NH 3 + H 2 O NH 4+ + OH-
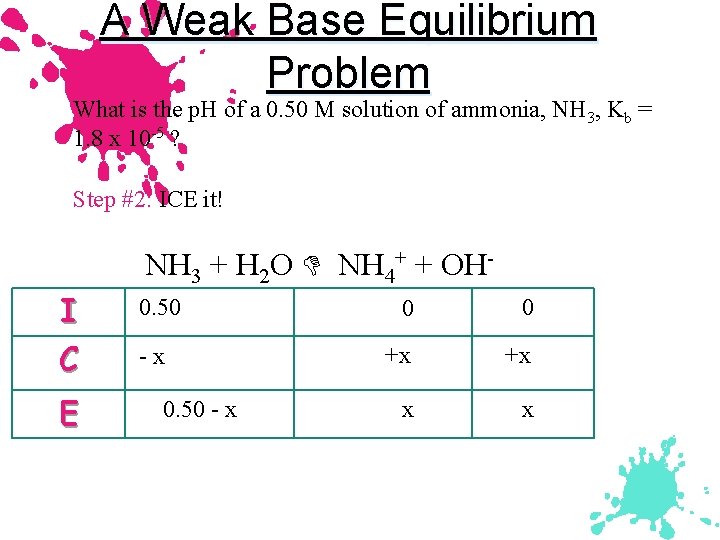
A Weak Base Equilibrium Problem What is the p. H of a 0. 50 M solution of ammonia, NH 3, Kb = 1. 8 x 10 -5 ? Step #2: ICE it! I C E NH 3 + H 2 O NH 4+ + OH 0. 50 -x 0. 50 - x 0 0 +x +x x x
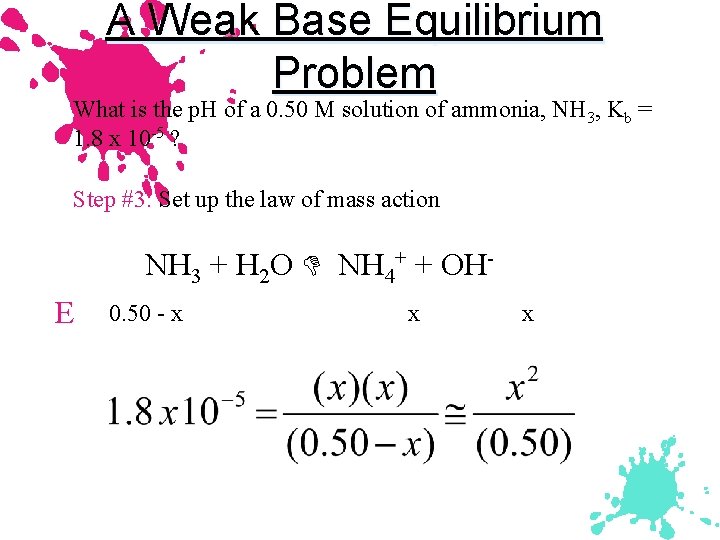
A Weak Base Equilibrium Problem What is the p. H of a 0. 50 M solution of ammonia, NH 3, Kb = 1. 8 x 10 -5 ? Step #3: Set up the law of mass action NH 3 + H 2 O NH 4+ + OHE 0. 50 - x x x

A Weak Base Equilibrium Problem What is the p. H of a 0. 50 M solution of ammonia, NH 3, Kb = 1. 8 x 10 -5 ? Step #4: Solve for x, which is also [OH-] NH 3 + H 2 O NH 4+ + OHE 0. 50 - x x x [OH-] = 3. 0 x 10 -3 M
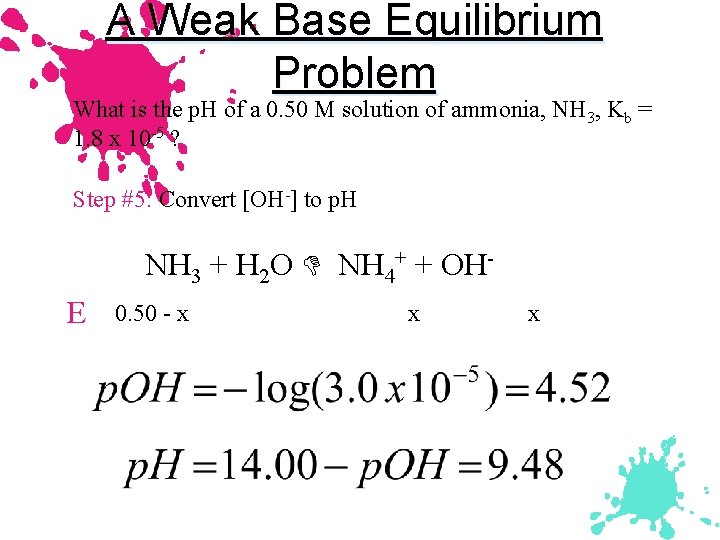
A Weak Base Equilibrium Problem What is the p. H of a 0. 50 M solution of ammonia, NH 3, Kb = 1. 8 x 10 -5 ? Step #5: Convert [OH-] to p. H NH 3 + H 2 O NH 4+ + OHE 0. 50 - x x x

Polyprotic Acids that can furnish more than one proton. Always dissociates in a stepwise manner, one proton at a time. The conjugate base of the first dissociation equilibrium becomes the acid in the second step. For a typical weak polyprotic acid: Ka 1 > Ka 2 > Ka 3 For a typical polyprotic acid in water, only the FIRST dissociation step is important to p. H.
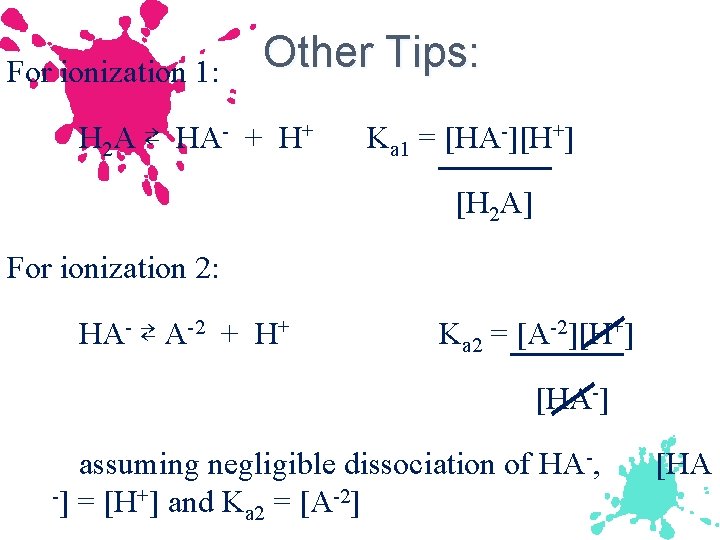
For ionization 1: Other Tips: H 2 A ⇄ HA- + H+ Ka 1 = [HA-][H+] [H 2 A] For ionization 2: HA- ⇄ A-2 + H+ Ka 2 = [A-2][H+] [HA-] assuming negligible dissociation of HA-, -] = [H+] and K = [A-2] a 2 [HA
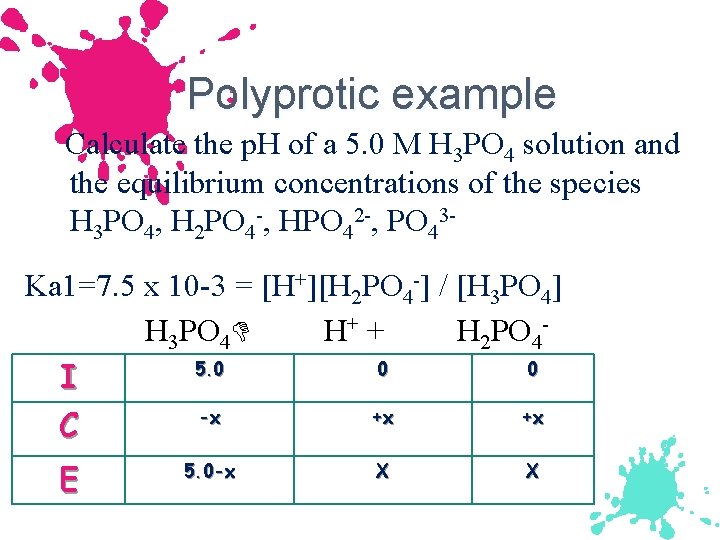
Polyprotic example Calculate the p. H of a 5. 0 M H 3 PO 4 solution and the equilibrium concentrations of the species H 3 PO 4, H 2 PO 4 -, HPO 42 -, PO 43 Ka 1=7. 5 x 10 -3 = [H+][H 2 PO 4 -] / [H 3 PO 4] H 3 PO 4 H+ + H 2 PO 45. 0 0 0 I -x +x +x C E 5. 0 -x X X
![7. 5 x 10 -3 = [x][x]/[5. 0 -x] 7. 5 x 10 -3 7. 5 x 10 -3 = [x][x]/[5. 0 -x] 7. 5 x 10 -3](http://slidetodoc.com/presentation_image_h2/1f550dca989e533643fe96a246086379/image-79.jpg)
7. 5 x 10 -3 = [x][x]/[5. 0 -x] 7. 5 x 10 -3 = [x][x]/[5. 0] x= 1. 9 x 10 -1 (check 5% rule) [H+] = 0. 19 M, p. H = 0. 72
![[H+]=[H 2 PO 4 -]=0. 19 M [H 3 PO 4] = 5. 0 [H+]=[H 2 PO 4 -]=0. 19 M [H 3 PO 4] = 5. 0](http://slidetodoc.com/presentation_image_h2/1f550dca989e533643fe96a246086379/image-80.jpg)
[H+]=[H 2 PO 4 -]=0. 19 M [H 3 PO 4] = 5. 0 – 0. 19 = 4. 8 M NOW USE OTHER Ka’s to solve for other [ ] Ka 2=6. 2 x 10 -8 = [H+][HPO 42 -] / [H 2 PO 4 -] = 6. 2 x 10 -8 = [. 19][HPO 42 -] / [. 19] = 6. 2 x 10 -8 M = [HPO 42 -]
![Ka 3= 4. 8 x 10 -13 = [H+][PO 43 -] / [HPO 42 Ka 3= 4. 8 x 10 -13 = [H+][PO 43 -] / [HPO 42](http://slidetodoc.com/presentation_image_h2/1f550dca989e533643fe96a246086379/image-81.jpg)
Ka 3= 4. 8 x 10 -13 = [H+][PO 43 -] / [HPO 42 -] 4. 8 x 10 -13 = [. 19][PO 43 -] / [6. 2 x 10 -8] [PO 43 -] = [(4. 8 x 10 -13 )(6. 2 x 10 -8)] /. 19 [PO 43 -] = 1. 6 x 10 -19 M
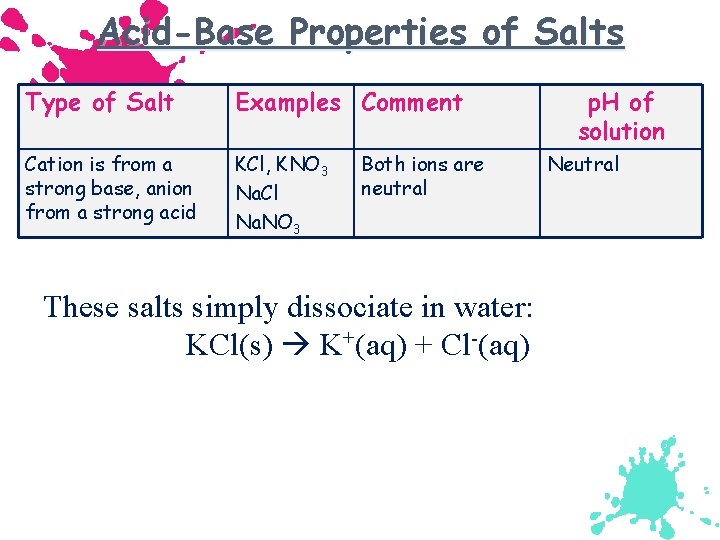
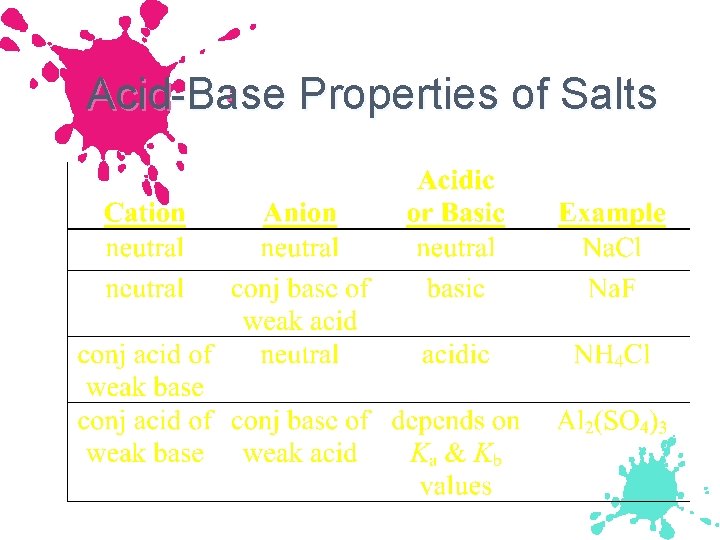
Acid-Base Properties of Salts Type of Salt Examples Comment Cation is from a strong base, anion from a strong acid KCl, KNO 3 Na. Cl Na. NO 3 Both ions are neutral These salts simply dissociate in water: KCl(s) K+(aq) + Cl-(aq) p. H of solution Neutral
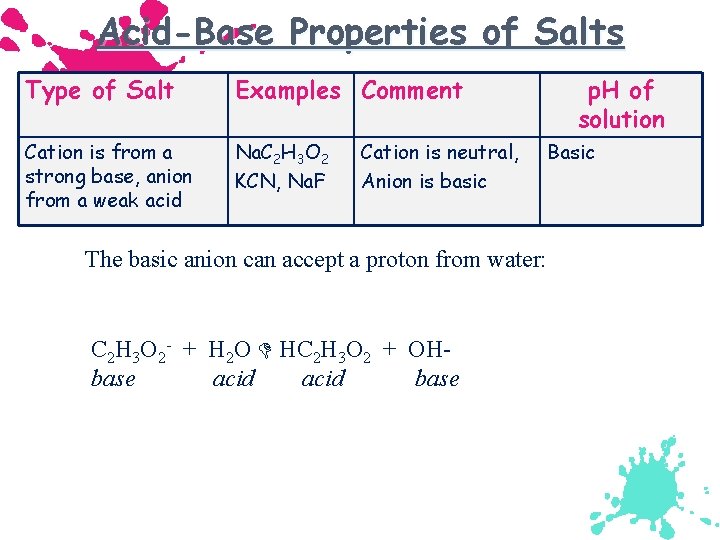
Acid-Base Properties of Salts Type of Salt Examples Comment Cation is from a strong base, anion from a weak acid Na. C 2 H 3 O 2 KCN, Na. F Cation is neutral, Anion is basic p. H of solution Basic The basic anion can accept a proton from water: C 2 H 3 O 2 - + H 2 O HC 2 H 3 O 2 + OHbase acid base
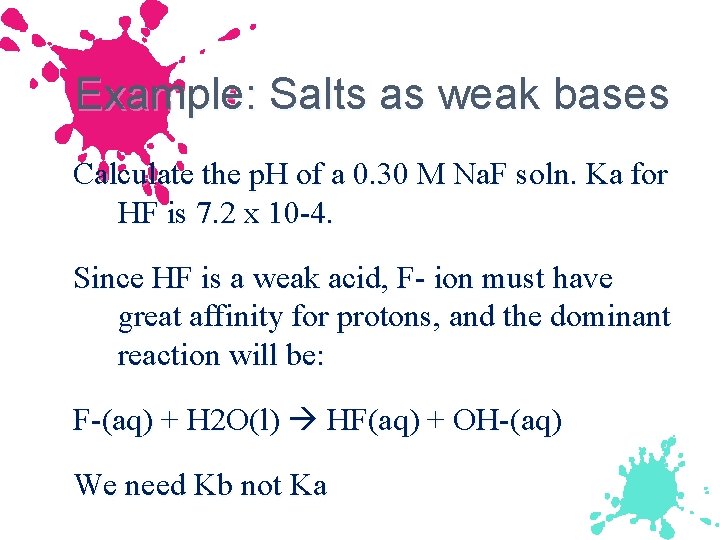
Example: Salts as weak bases Calculate the p. H of a 0. 30 M Na. F soln. Ka for HF is 7. 2 x 10 -4. Since HF is a weak acid, F- ion must have great affinity for protons, and the dominant reaction will be: F-(aq) + H 2 O(l) HF(aq) + OH-(aq) We need Kb not Ka
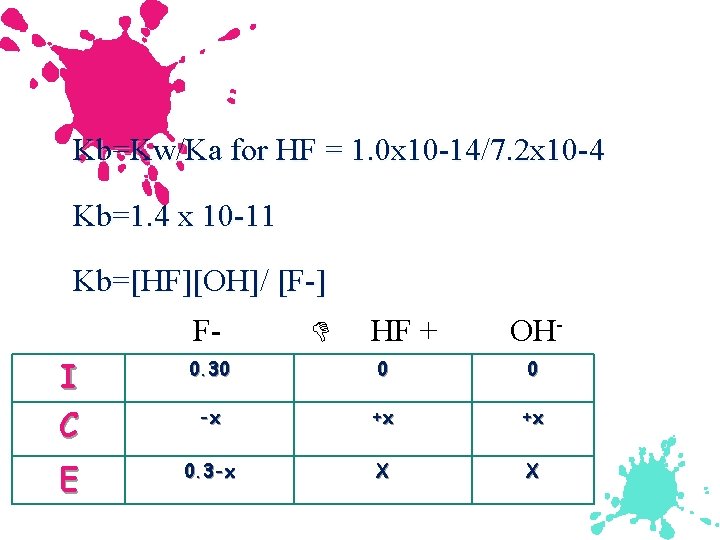
Kb=Kw/Ka for HF = 1. 0 x 10 -14/7. 2 x 10 -4 Kb=1. 4 x 10 -11 Kb=[HF][OH]/ [F-] F- HF + OH- I C 0. 30 0 0 -x +x +x E 0. 3 -x X X
![Kb=1. 4 x 10 -11=[x][x]/ [. 30 -x] Kb=1. 4 x 10 -11=[x][x]/ [. Kb=1. 4 x 10 -11=[x][x]/ [. 30 -x] Kb=1. 4 x 10 -11=[x][x]/ [.](http://slidetodoc.com/presentation_image_h2/1f550dca989e533643fe96a246086379/image-86.jpg)
Kb=1. 4 x 10 -11=[x][x]/ [. 30 -x] Kb=1. 4 x 10 -11=[x][x]/ [. 30] x=2. 0 x 10 -6 [OH]=2. 0 x 10 -6 p. OH=5. 69 p. H=14 -5. 69=8. 31
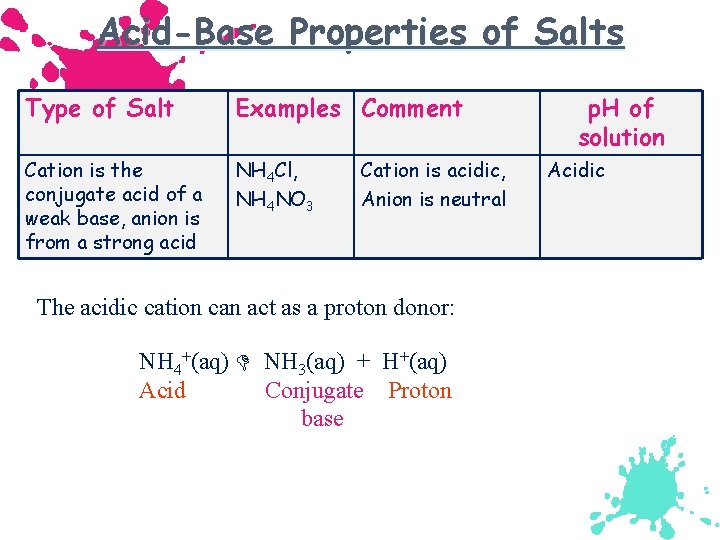
Acid-Base Properties of Salts Type of Salt Examples Comment Cation is the conjugate acid of a weak base, anion is from a strong acid NH 4 Cl, NH 4 NO 3 Cation is acidic, Anion is neutral The acidic cation can act as a proton donor: NH 4+(aq) NH 3(aq) + H+(aq) Acid Conjugate Proton base p. H of solution Acidic

Example: Salts as weak acids Calculate the p. H of a 0. 10 M NH 4 Cl soln. Kb for NH 3 is 1. 8 x 10 -5. Dominant reaction will be: NH 4+(aq) NH 3(aq) + H+(aq) We need Ka not Kb
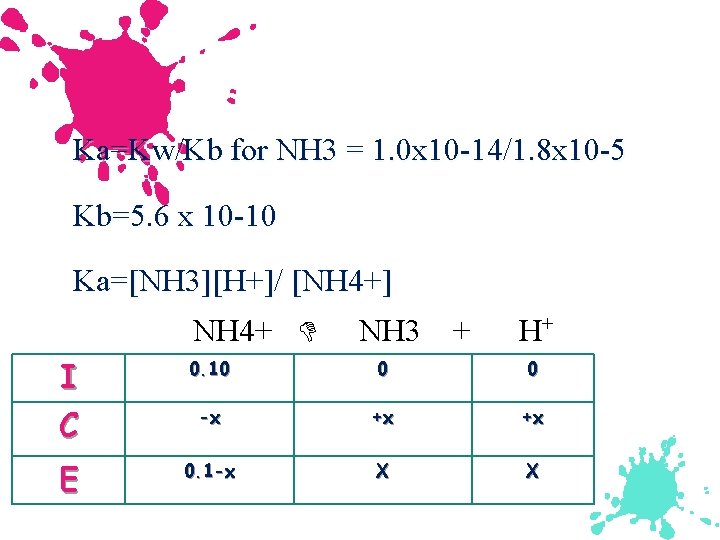
Ka=Kw/Kb for NH 3 = 1. 0 x 10 -14/1. 8 x 10 -5 Kb=5. 6 x 10 -10 Ka=[NH 3][H+]/ [NH 4+] NH 4+ NH 3 + H+ I C 0. 10 0 0 -x +x +x E 0. 1 -x X X
![Ka=5. 6 x 10 -10=[x][x]/ [. 10 -x] Ka=5. 6 x 10 -10=[x][x]/ [. Ka=5. 6 x 10 -10=[x][x]/ [. 10 -x] Ka=5. 6 x 10 -10=[x][x]/ [.](http://slidetodoc.com/presentation_image_h2/1f550dca989e533643fe96a246086379/image-90.jpg)
Ka=5. 6 x 10 -10=[x][x]/ [. 10 -x] Ka=5. 6 x 10 -10=[x][x]/ [. 10] x=7. 6 x 10 -6 [H+]=2. 0 x 10 -6 p. H=5. 13
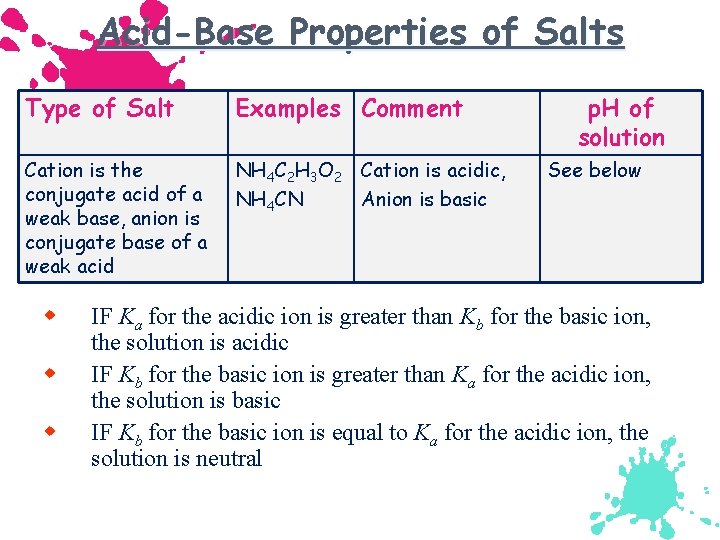
Acid-Base Properties of Salts Type of Salt Examples Comment Cation is the conjugate acid of a weak base, anion is conjugate base of a weak acid NH 4 C 2 H 3 O 2 Cation is acidic, NH 4 CN Anion is basic w w w p. H of solution See below IF Ka for the acidic ion is greater than Kb for the basic ion, the solution is acidic IF Kb for the basic ion is greater than Ka for the acidic ion, the solution is basic IF Kb for the basic ion is equal to Ka for the acidic ion, the solution is neutral
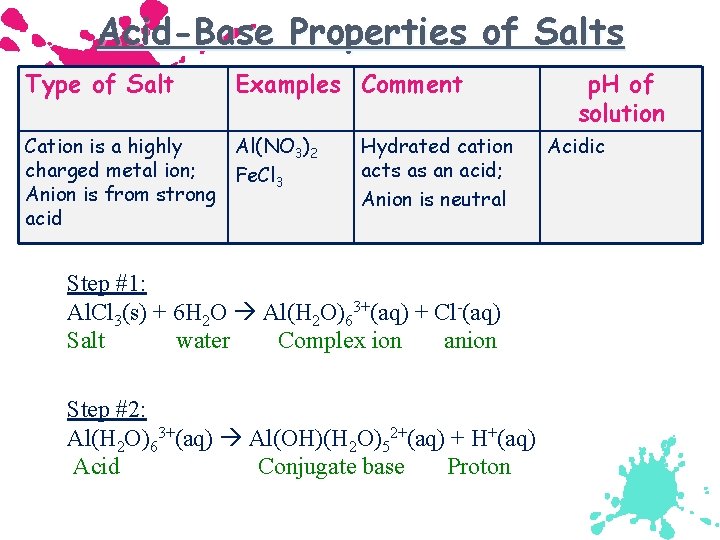
Acid-Base Properties of Salts Type of Salt Examples Comment Cation is a highly Al(NO 3)2 charged metal ion; Fe. Cl 3 Anion is from strong acid Hydrated cation acts as an acid; Anion is neutral Step #1: Al. Cl 3(s) + 6 H 2 O Al(H 2 O)63+(aq) + Cl-(aq) Salt water Complex ion anion Step #2: Al(H 2 O)63+(aq) Al(OH)(H 2 O)52+(aq) + H+(aq) Acid Conjugate base Proton p. H of solution Acidic
Acid-Base Properties of Salts
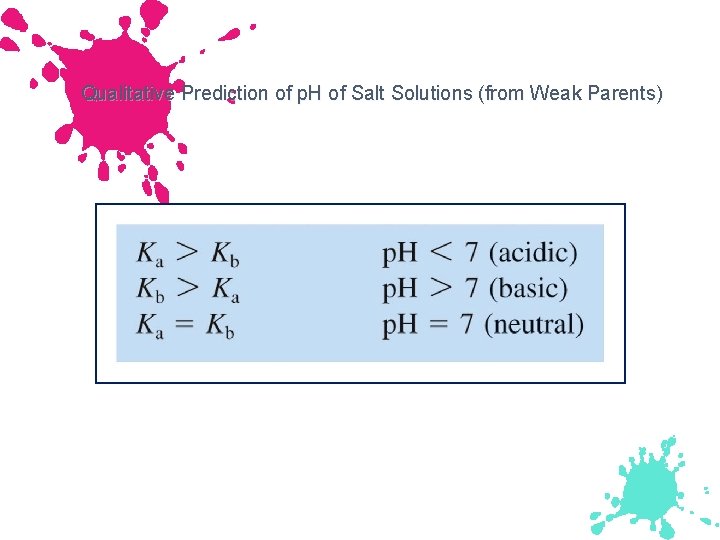
Qualitative Prediction of p. H of Salt Solutions (from Weak Parents)

Example Predict whether an aqueous solution of each of the following will be acidic, basic, or neutral a. NH 4 C 2 H 3 O 2: b. NH 4 CN: c. Al 2(SO 4)3:

Example Predict whether an aqueous solution of each of the following will be acidic, basic, or neutral a. NH 4 C 2 H 3 O 2: Ka NH 4+ = Kb C 2 H 3 O 2 -, neutral b. NH 4 CN: Ka NH 4+ < Kb CN- , Basic c. Al 2(SO 4)3: Ka Al 2(H 2 O)63+ > Kb SO 42 -, Acidic
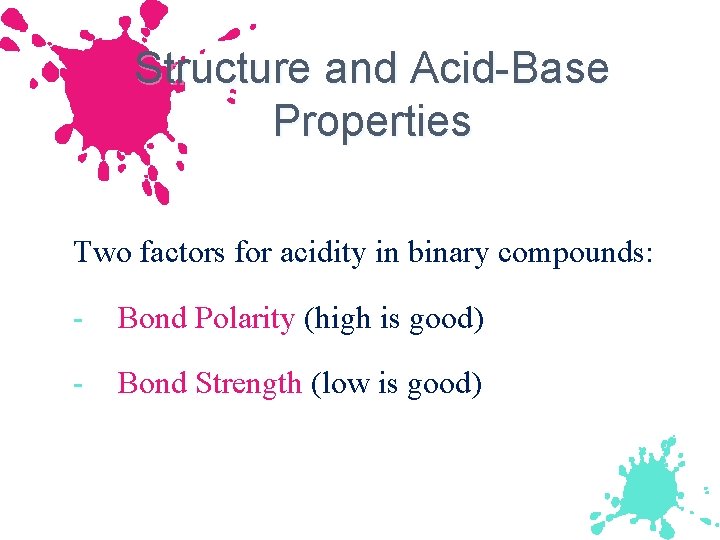
Structure and Acid-Base Properties Two factors for acidity in binary compounds: - Bond Polarity (high is good) - Bond Strength (low is good)
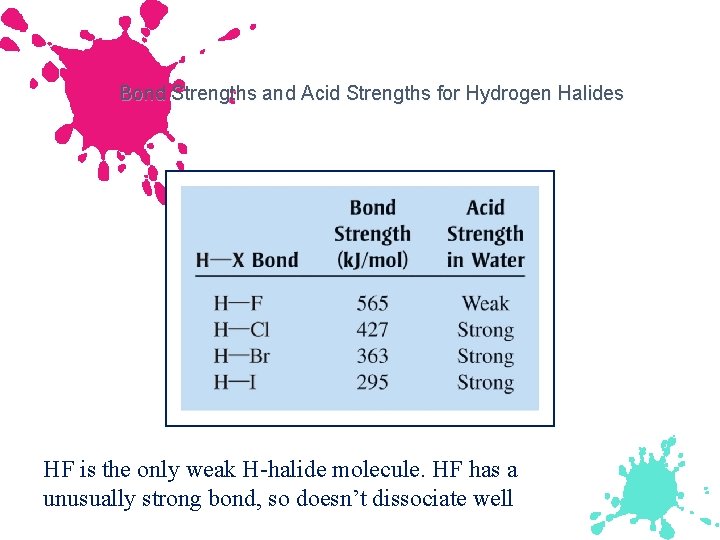
Bond Strengths and Acid Strengths for Hydrogen Halides HF is the only weak H-halide molecule. HF has a unusually strong bond, so doesn’t dissociate well

Oxyacids Contains the group H–O–X. For a given series the acid strength increases with an increase in the number of oxygen atoms attached to the central atom. The greater the ability of X to draw electrons toward itself, the greater the acidity of the molecule.
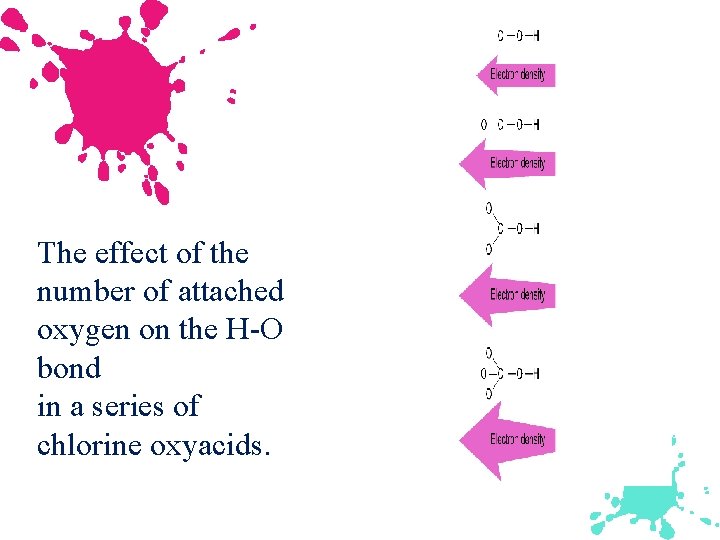
The effect of the number of attached oxygen on the H-O bond in a series of chlorine oxyacids.
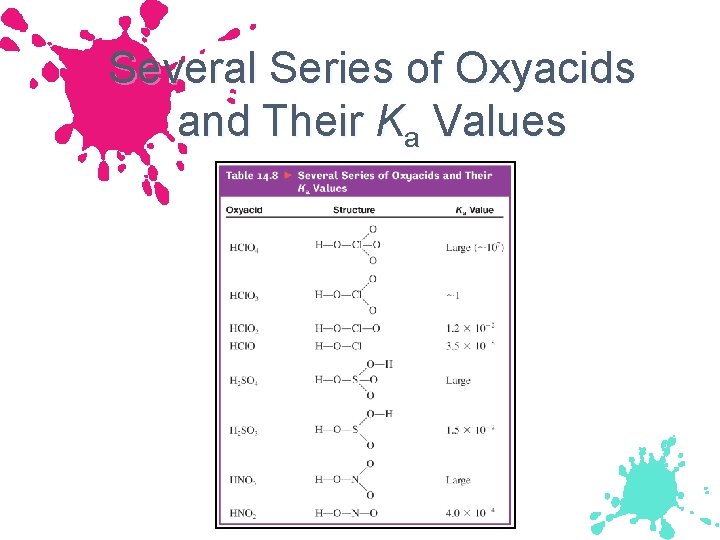
Several Series of Oxyacids and Their Ka Values
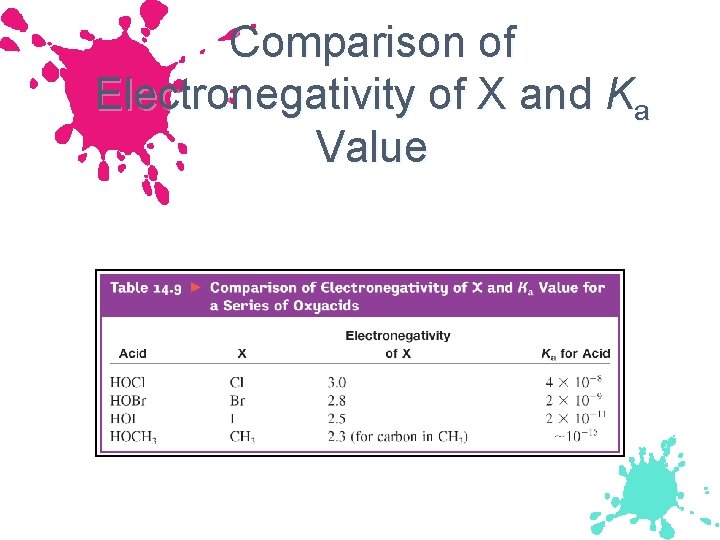
Comparison of Electronegativity of X and Ka Value

Oxides Acidic Oxides (Acid Anhydrides): - O X bond is strong and covalent. SO 2, NO 2, Cr. O 3 Basic Oxides (Basic Anhydrides): - O X bond is ionic. K 2 O, Ca. O

Oxides Acidic Oxides (Acid Anhydrides): § O-X bond is strong and covalent. SO 2, NO 2, CO 2 When H-O-X grouping is dissolved in water, the O-X bond will remain intact. It will be the polar and relatively weak H-O bond that will tend to break, releasing a proton.

Oxides Basic Oxides (Basic Anhydrides): § O-X bond is ionic. K 2 O, Ca. O If X has a very low electronegativity, the O-X bond will be ionic and subject to being broken in polar water, producing a basic solution.
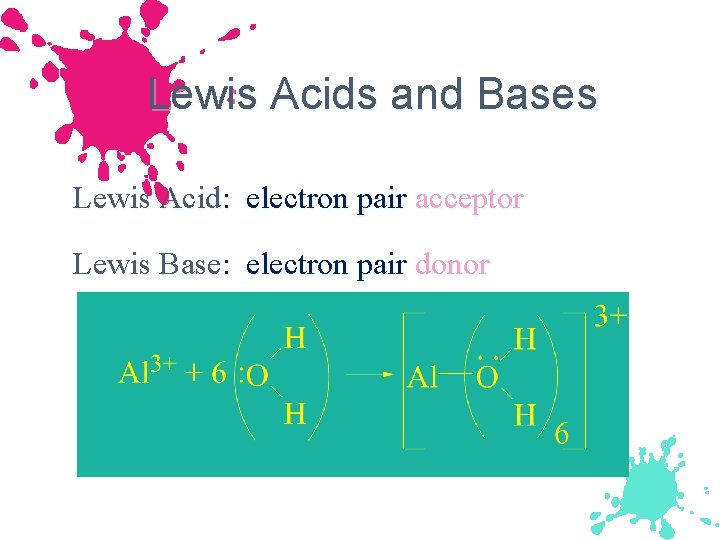
Lewis Acids and Bases Lewis Acid: electron pair acceptor Lewis Base: electron pair donor
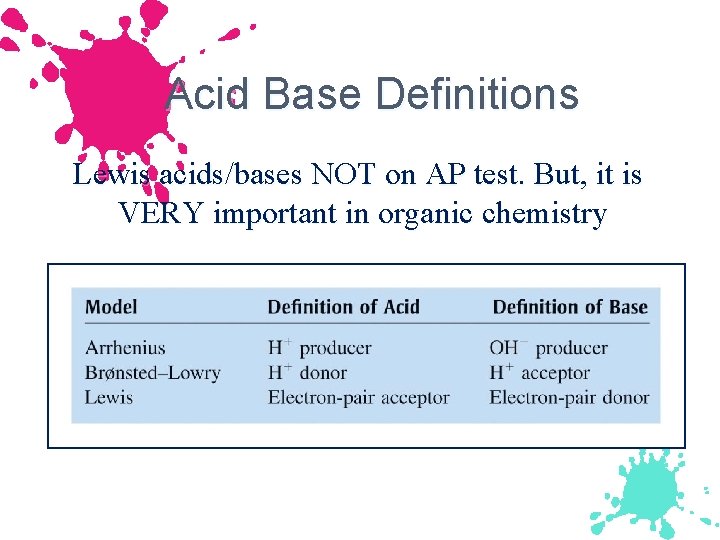
Acid Base Definitions Lewis acids/bases NOT on AP test. But, it is VERY important in organic chemistry
- Slides: 107