Acids and Bases All substances can be divided

Acids and Bases All substances can be divided up into groups of acids, acids bases and neutral substances.

Acids • Acids are corrosive, sour tasting chemicals • They release hydrogen ions in water (H+) • It is the reaction of hydrogen ions that make up the typical reactions of acids.

Properties of Acids • They react with metals to release hydrogen ions • • Change colour of indicators like litmus They react with metal carbonates and bicarbonates to release carbon dioxide gas • They neutralise bases to form salt and water • They provide H+ during chemical reactions

Acids found in nature –organic acids these are generally quite safe

Strong acid are corrosive and can eat away at metals and fabric – mineral acids Examples of acids at school • HCl hydrochloric acid • H 2 SO 4 sulfuric acid • HNO 3 nitric acid • Strong acids can be diluted with water

Other acids q Carbon dioxides dissolves in water – carbonic acid found in fizzy drinks H 2 CO 3 q. Non-metal oxides dissolve in water to form acidic solution eg sulfur dioxide – sulfurous acid

Bases • Bases are corrosive, bitter tasting chemicals • If they are soluble in water they are called alkalis • They release hydroxide ions (OH-) when dissolved and these are responsible for the properties of bases.

Properties of Bases. . . Neutralise acids to form salt and water They have a slippery feel • They react with fats and oils to produce soaps

Bases q metal oxides and hydroxides are examples of bases q Na. OH - sodium hydroxide, KOH potassium hydroxide, NH 4 OH ammonium hydroxide , calcium hydroxide Ca(OH)2 common alkalis found in the lab q. Bases ionises in water ( breakup) producing hydroxide ions OHq. Metal oxides are bases and metal hydoxides q. Soluble bases are called alkalis

p. H scale We can determine the strength of an acid or base using the numbers 0 – 14 on the p. H scale. Strong acids are nearer 0 Neutral is 7 Strong bases are nearer 14 p. H is a measure of H+ ion concentration in a solution The higher the H+ concentration the smaller the p. H valve and the more acidic the solution is.
Indicators Whether a substance is an acid, base or neutral can also be shown by its reaction with indicators These change colour according to how acidic or basic the substance is.
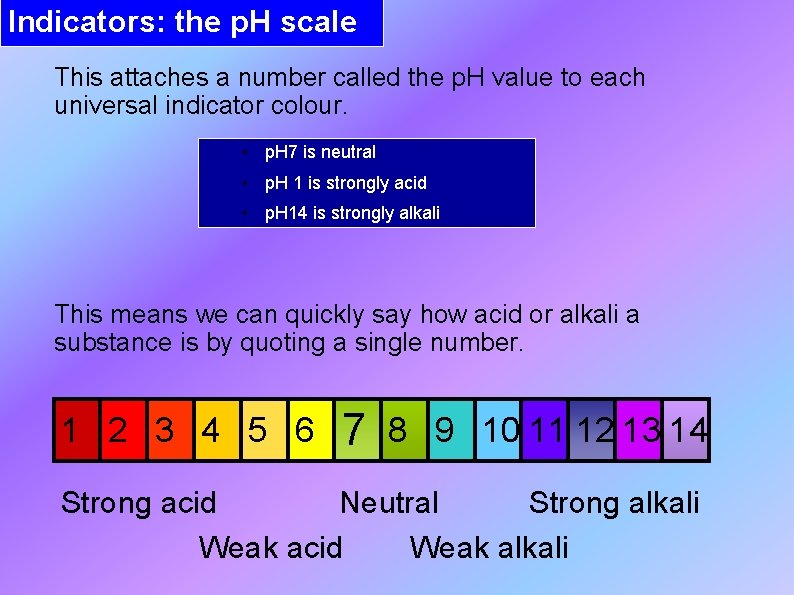
Indicators: the p. H scale This attaches a number called the p. H value to each universal indicator colour. • p. H 7 is neutral • p. H 1 is strongly acid • p. H 14 is strongly alkali This means we can quickly say how acid or alkali a substance is by quoting a single number. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Strong acid Neutral Strong alkali Weak acid Weak alkali

Practical. . . Aim: To test a range of substances with indicators to see how strong an acid or a base they are. Method: Add 2 drops of universal indicator solution to a range of solutions in spotting trays. Use the universal indicator colour chart to identify the p. H. Complete the table on page 56 in your work book

Litmus Paper Litmus paper can be red or blue. Litmus paper remains (or turns red) in acids. Litmus paper remains (or turns blue) in bases.
- Slides: 14