Acids and Bases Acids Bases Acids 1 Produce

Acids and Bases

Acids & Bases Acids 1. Produce H+ ions in water forming H 3 O+ (hydronium) 2. Taste sour 3. Corrosive 4. React with metals to produce H 2 gas 5. Turn litmus RED Bases 1. Produce OHions in water 2. Taste bitter 3. Corrosive 4. Feel slippery 5. Turn litmus BLUE
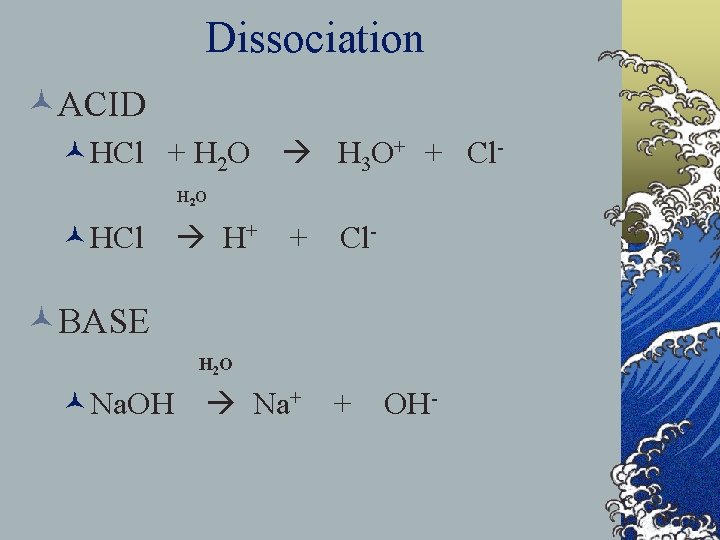
Dissociation ©ACID ©HCl + H 2 O H 3 O+ + Cl. H 2 O ©HCl H+ + Cl- ©BASE H 2 O ©Na. OH Na+ + OH-

Acids & Bases ©Strong acids ionizes almost completely in solution ( ) i. e. HCl & H 2 SO 4 ©Strong Electrolyte ©Weak acids ionizes partially in solution ( or ) i. e. HCl O & H 2 CO 3 ©Weak Electrolyte

Acids & Bases ©Strong bases dissociate completely in solution ( ) i. e. KOH & Na. OH ©Weak bases dissociate partially in solution ( or ) i. e. NH 3
Acids & Bases ©Dilute and concentrated are terms to describe the amount of acid or base dissolved. ©p. H – measure of H+ concentration in solution ©Buffers is a solution that resist change in p. H (i. e. blood has HCO 2 as a buffer to keep it at a near constant p. H of 7. 4)
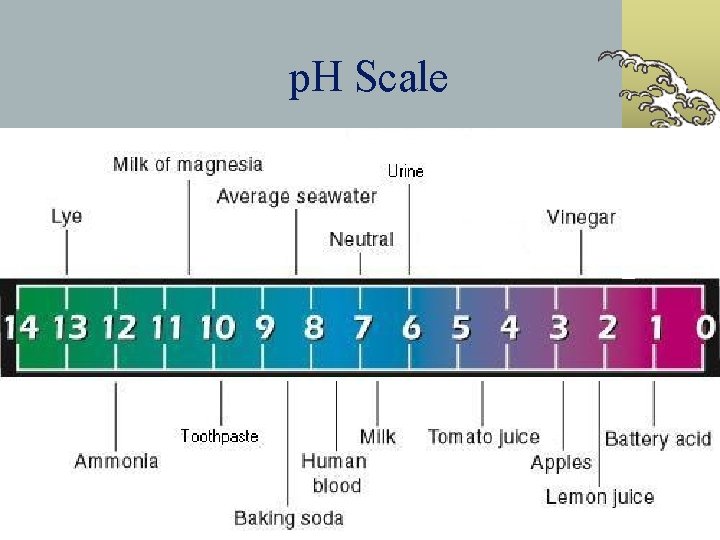
p. H Scale

Acids & Bases ©Neutralization is the chemical reaction between an acid and a base taking place in a water solution ©Acid + Base Salt + Water ©Titration is the process used to determine the concentration of an acidic or basic solution
Acids & Bases ©End point – neutralization point ©Indicator is an organic compound that changes color in acids & bases (i. e. litmus paper)
- Slides: 9