Acids and Bases Acids An acid is compound

Acids and Bases

Acids An acid is compound that produces hydrogen ions (H+) when dissolved in water. Naming acids If anion ends in –ide, the acid name begins w/ hydro- and ends w/ -ic. Example HCl Hydrochloric Acid If anion ends in –ite, the acid name is the stem of the anion w/ the suffix –ous. Example H 2 SO 3 Sulfurous Acid
Cont. If the anion ends in –ate, the acid name is the stem of the anion w/ the suffix –ic. Example HNO 3 Nitric Acid

Bases A base is a compound that produces hydroxide ions (OH-) when dissolved in water. Naming bases Bases are named the same as any other ionic compound. Example Na. OH Sodium hydroxide
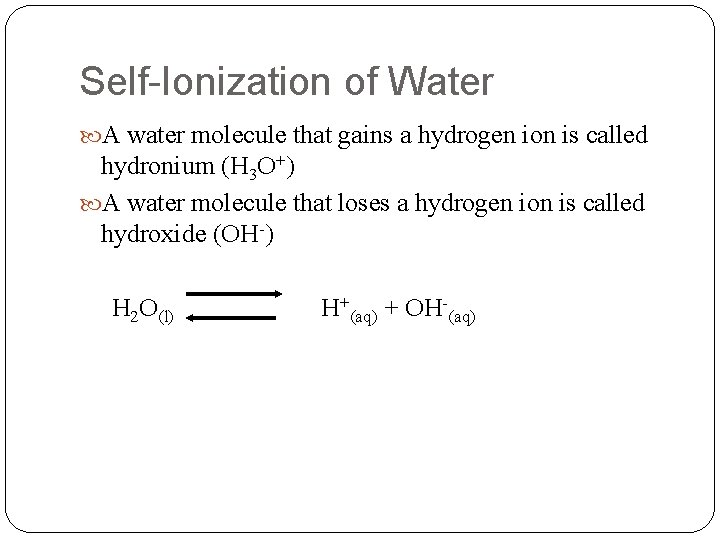
Self-Ionization of Water A water molecule that gains a hydrogen ion is called hydronium (H 3 O+) A water molecule that loses a hydrogen ion is called hydroxide (OH-) H 2 O(l) H+(aq) + OH-(aq)

Cont. The rxn in which 2 water molecules produce ions is called the self-ionization of water. In pure water the concentration of hydrogen ions and the concentration of hydroxide ions are each 1. 0 x 107 M If H+ decreases, then OH- increases, and vice versa.
![Ion-product Constant for Water Kw Kw = [H+] x [OH-] = 1. 0 x Ion-product Constant for Water Kw Kw = [H+] x [OH-] = 1. 0 x](http://slidetodoc.com/presentation_image_h2/a50e0c9ff19fbe322c59dfc8ecac358c/image-7.jpg)
Ion-product Constant for Water Kw Kw = [H+] x [OH-] = 1. 0 x 10 -14 M When some substances dissolve in water they release hydrogen ions (Acids) When some substances dissolve in water they release hydroxide ions (Bases)
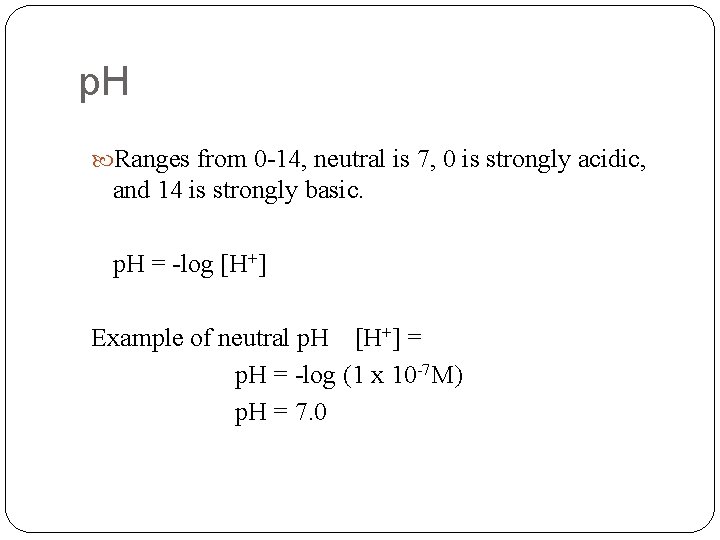
p. H Ranges from 0 -14, neutral is 7, 0 is strongly acidic, and 14 is strongly basic. p. H = -log [H+] Example of neutral p. H [H+] = p. H = -log (1 x 10 -7 M) p. H = 7. 0
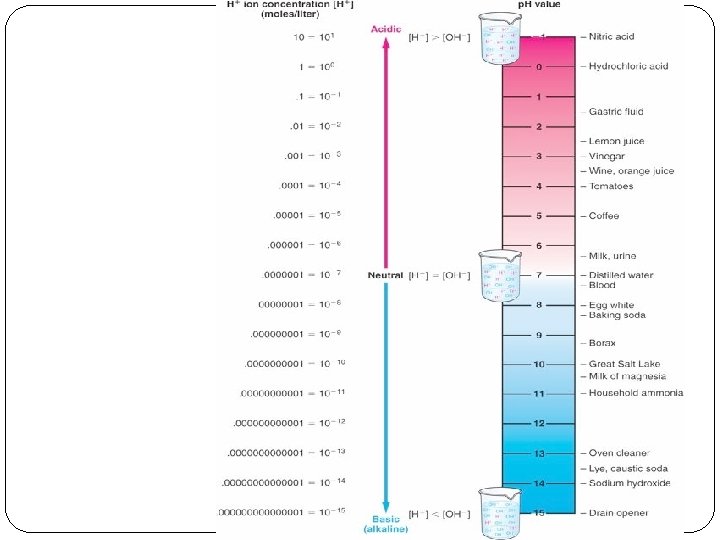
Measuring p. H Acid-Base indicators – indicators are acids or bases that undergo dissociation in a known p. H range. When it undergoes dissociation there is a color change Examples Thymol blue Bromthymol blue Phenolphthalein p. H meters - record electric activity using a millivoltmeter.

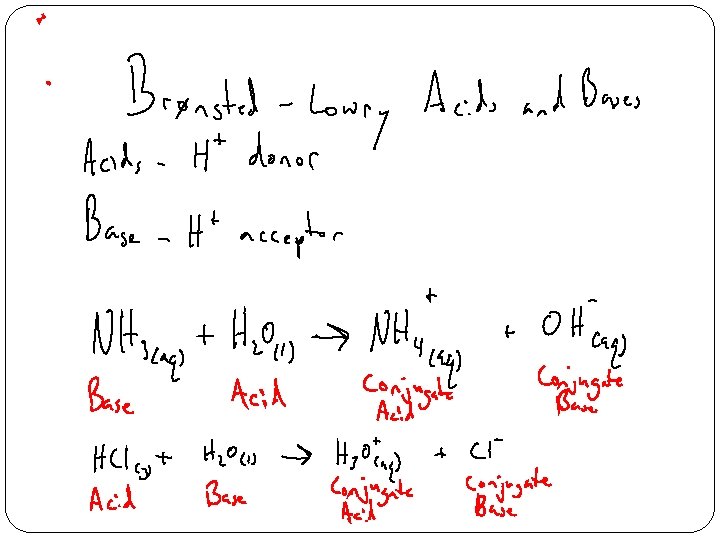
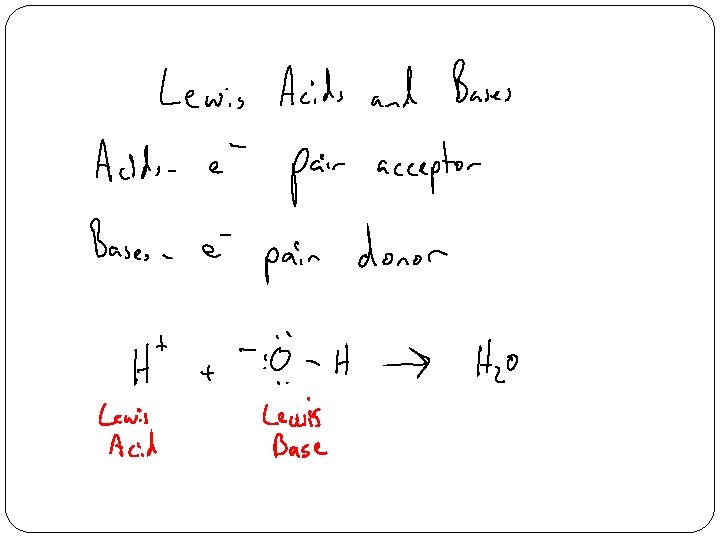


- Slides: 15