ACIDS AND BASES Acids Acidic solutions contain water

ACIDS AND BASES

Acids Acidic solutions contain water and hydrogen ions (H+)

Acids Acidic solutions contain water and hydrogen ions (H+) When dilute acidic solutions react, it is the hydrogen ions that are involved in the reaction.

Acids - Acidic solutions contain water and hydrogen ions (H+) When dilute acidic solutions react, it is the hydrogen ions that are involved in the reaction. Common acids are: Hydrochloric acid (HCl)

Acids - Acidic solutions contain water and hydrogen ions (H+) When dilute acidic solutions react, it is the hydrogen ions that are involved in the reaction. Common acids are: Hydrochloric acid (HCl) Nitric Acid (HNO 3)

Acids - Acidic solutions contain water and hydrogen ions (H+) When dilute acidic solutions react, it is the hydrogen ions that are involved in the reaction. Common acids are: Hydrochloric acid (HCl) Nitric Acid (HNO 3) Sulfuric Acid (H 2 SO 4)

Acids - Acidic solutions contain water and hydrogen ions (H+) When dilute acidic solutions react, it is the hydrogen ions that are involved in the reaction. Common acids are: Hydrochloric acid (HCl) Nitric Acid (HNO 3) Sulfuric Acid (H 2 SO 4) Citrus fruits are acidic, as well as carbonated drinks (like coke).

Bases are substances that neutralise acids by forming water and a salt.

Bases - Bases are substances that neutralise acids by forming water and a salt. Common bases are: Sodium Hydroxide (Na. OH)

Bases - Bases are substances that neutralise acids by forming water and a salt. Common bases are: Sodium Hydroxide (Na. OH) Calcium hydroxide ( Ca(OH)2 ) or limewater

Bases - Bases are substances that neutralise acids by forming water and a salt. Common bases are: Sodium Hydroxide (Na. OH) Calcium hydroxide ( Ca(OH)2 ) or limewater Ammonia solution (NH 3)

Bases Bases are substances that neutralise acids by forming water and a salt. Common bases are: Sodium Hydroxide (Na. OH) Calcium hydroxide ( Ca(OH)2 ) or limewater Ammonia solution (NH 3) Bases have hydroxide ions (OH-)

Neutral Substances Some substances, such as salt (Sodium Chloride) dissolve in water, forming solutions which are neutral.

Neutral Substances Some substances, such as salt (Sodium Chloride) dissolve in water forming solutions which are neutral. Pure water is neutral
The p. H scale Some acids are stronger than others; strong acids produce the highest concentration of hydrogen ions.
The p. H scale Some acids are stronger than others; strong acids produce the highest concentration of hydrogen ions. Strong bases produce high concentrations of hydroxide ions.
The p. H scale Some acids are stronger than others; strong acids produce the highest concentration of hydrogen ions. Strong bases produce high concentrations of hydroxide ions. The same applies for weak acids/weak bases having low concentrations of particular ions.
The p. H scale Some acids are stronger than others; strong acids produce the highest concentration of hydrogen ions. Strong bases produce high concentrations of hydroxide ions. The same applies for weak acids/weak bases having low concentrations of particular ions. The p. H scale is used to measure how acidic or basic a solution is.

The p. H scale Acidic solutions have p. H of less than 7

The p. H scale Acidic solutions have p. H of less than 7 Basic solutions have p. H of more than 7

The p. H scale Acidic solutions have p. H of less than 7 Basic solutions have p. H of more than 7 Neutral solutions are 7

The p. H scale Acidic solutions have p. H of less than 7 Basic solutions have p. H of more than 7 Neutral solutions are 7 Red and blue litmus paper can be used to indicate whether the solution is an acid or a base.

The p. H scale Acidic solutions have p. H of less than 7 Basic solutions have p. H of more than 7 Neutral solutions are 7 Red and blue litmus paper can be used to indicate whether the solution is an acid or a base. Red – turns blue in a base Blue – turns red in an acid
The p. H scale Pour 1 cm of solution into your 5 test tubes Using red or blue litmus paper, drop the paper into the test tubes and see what colours they change to. Write down what solution is an acid or base
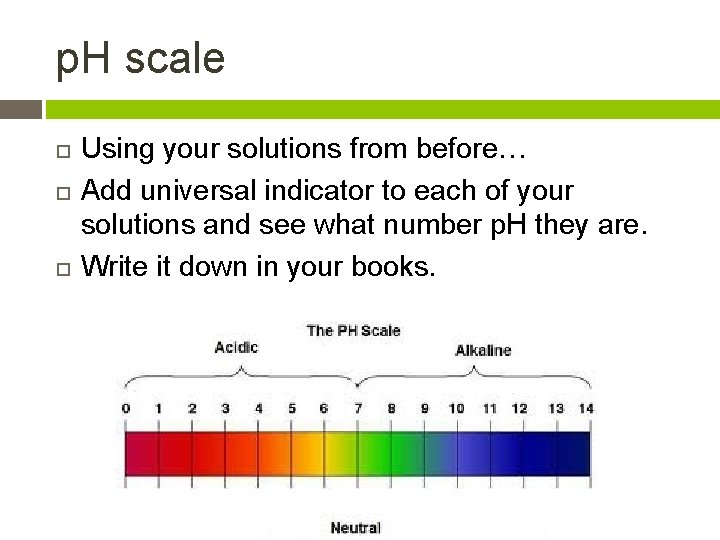
p. H scale Using your solutions from before… Add universal indicator to each of your solutions and see what number p. H they are. Write it down in your books.
- Slides: 25