Acids and Bases Acid Group of compounds with

Acids and Bases

Acid Group of compounds with some common properties when in solution n 1 st – taste sour n 2 nd – change the color of certain dyes called indicators n 3 rd – most react with active metals to release hydrogen gas n

4 th – acids react with bases to produce neutral salt and water n 5 th – acids conduct electric current n

Two Types of Acids Binary acid – contains only hydrogen and another element n Oxyacid -Acid with hydrogen, oxygen and a third element n

Acid Nomenclature n n n Name is based on the anion that it contains In the anion that makes up the acid ends in –ide, the acid starts with the prefix hydro- and ends with the suffix -ic If the anion ends in –ite, the acid ends in –ous If the anion ends in –ate, the acid ends in -ic HCl, HF, HI, HCl. O, HNO 2, HNO 3

Properties of Bases taste bitter n Feel slippery to the skin n Bases change color of indicators n React with acid to form water and a salt n Are electrolytes n Excellent cleaning agents n

Base Nomenclature Usually have hydroxide ions n Na. OH, Al(OH)3, Mg(OH)2 n

Ionization n Acids and Bases both ionize in water HCl H+ + Cl. H 2 SO 4 2 H+ + SO 42 Al(OH)3 Al 3+ + 3 OH-

Acid and Base Definitions

Arrhenius Definition n Acid forms H+ n Base forms OH-
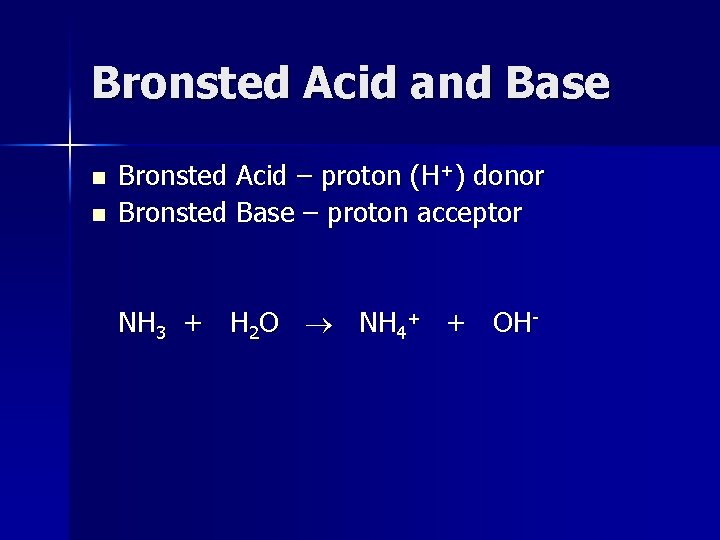
Bronsted Acid and Base n n Bronsted Acid – proton (H+) donor Bronsted Base – proton acceptor NH 3 + H 2 O NH 4+ + OH-
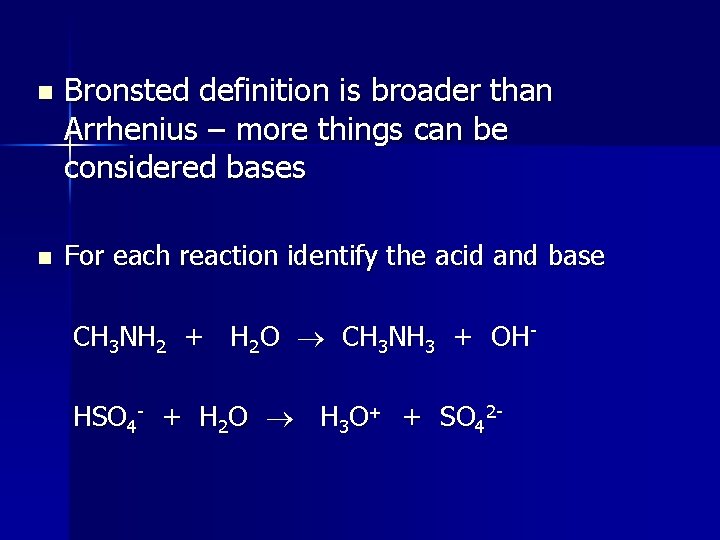
n n Bronsted definition is broader than Arrhenius – more things can be considered bases For each reaction identify the acid and base CH 3 NH 2 + H 2 O CH 3 NH 3 + OHHSO 4 - + H 2 O H 3 O+ + SO 42 -

Amphoteric In the last example we saw that water could act as either an acid or a base depending on what it reacted with n Water is amphoteric – can act as either an acid or a base n
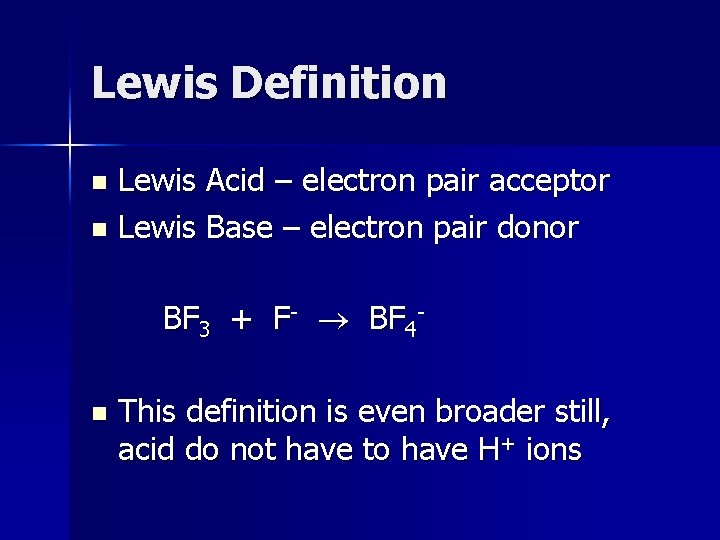
Lewis Definition Lewis Acid – electron pair acceptor n Lewis Base – electron pair donor n BF 3 + F- BF 4 n This definition is even broader still, acid do not have to have H+ ions

Neutralization Reaction between an acid and a base to form a salt and water n It is a double replacement reaction n

Example n Finish the reaction and identify the acid and base H 2 SO 4 + KOH
- Slides: 16