ACIDS AND BASES ACID BASE OR NEUTRAL Bitter
ACIDS AND BASES

ACID, BASE, OR NEUTRAL? Bitter taste base Doesn’t conduct electricity neutral Feels slippery base Dissolves metals acid Has no odor base or neutral Has a strong, acrid odor acid Turns litmus blue base Is unreactive neutral Feels sticky acid Feels greasy neutral Turns phenolphthalein solution red acid Dissolves grease base Bubbles when baking soda is added acid Is oily neutral
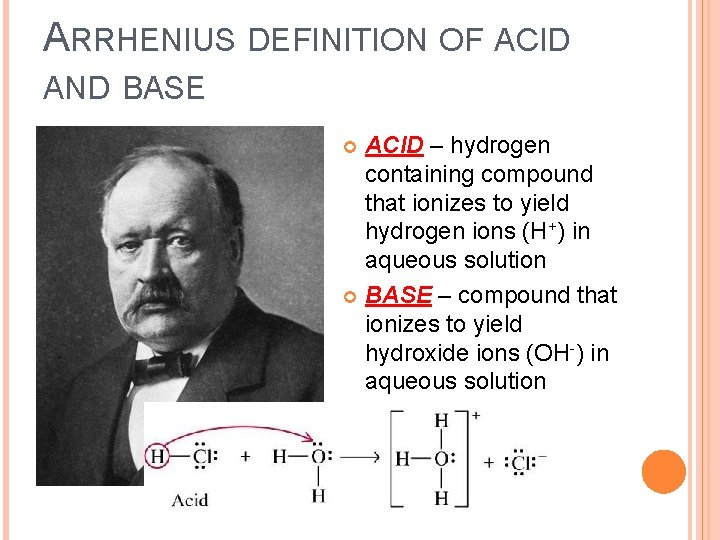
ARRHENIUS DEFINITION OF ACID AND BASE ACID – hydrogen containing compound that ionizes to yield hydrogen ions (H+) in aqueous solution BASE – compound that ionizes to yield hydroxide ions (OH-) in aqueous solution
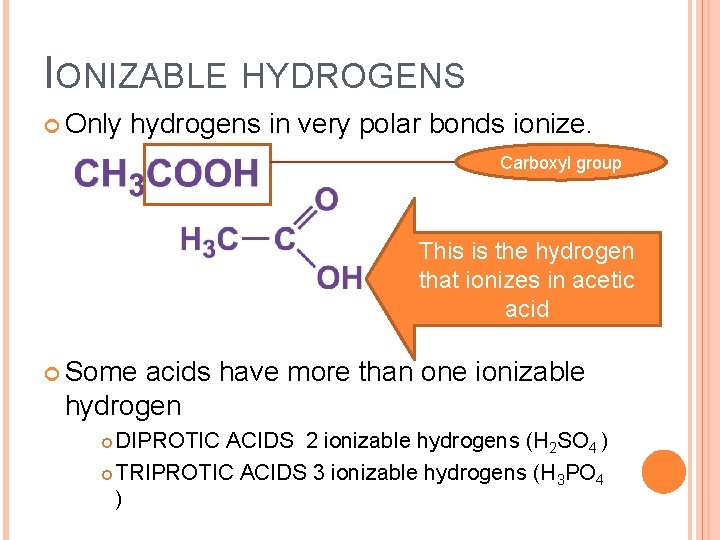
IONIZABLE HYDROGENS Only hydrogens in very polar bonds ionize. Carboxyl group This is the hydrogen that ionizes in acetic acid Some acids have more than one ionizable hydrogen DIPROTIC ACIDS 2 ionizable hydrogens (H 2 SO 4 ) TRIPROTIC ACIDS 3 ionizable hydrogens (H 3 PO 4 )
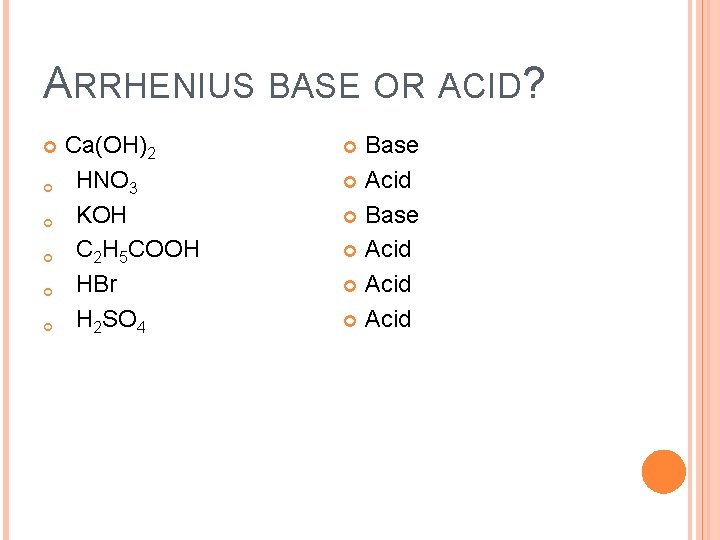
ARRHENIUS BASE OR ACID? Ca(OH)2 HNO 3 KOH C 2 H 5 COOH HBr H 2 SO 4 Base Acid
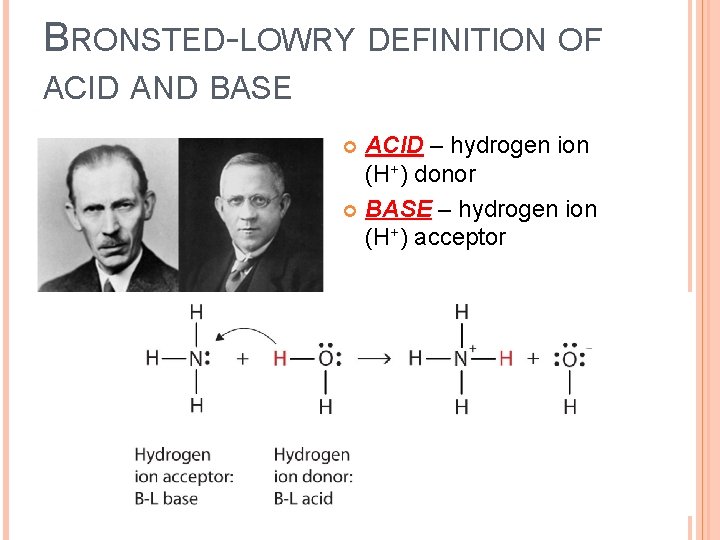
BRONSTED-LOWRY DEFINITION OF ACID AND BASE ACID – hydrogen ion (H+) donor BASE – hydrogen ion (H+) acceptor
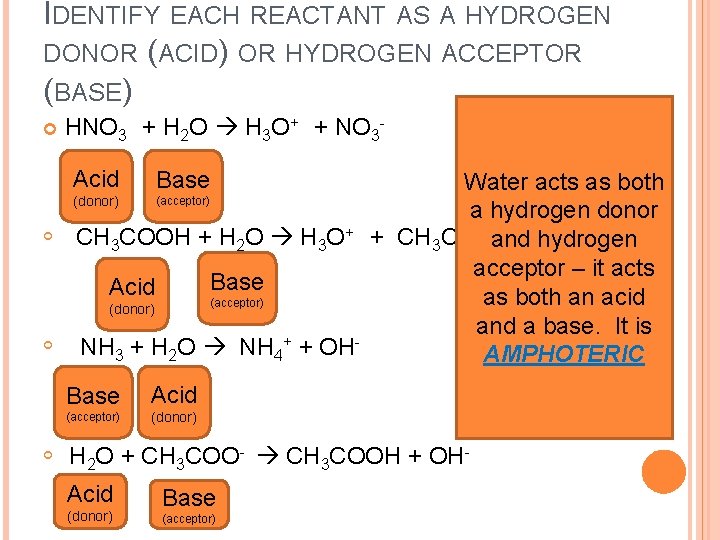
IDENTIFY EACH REACTANT AS A HYDROGEN DONOR (ACID) OR HYDROGEN ACCEPTOR (BASE) HNO 3 + H 2 O H 3 O+ + NO 3 Acid Base Acid (acceptor) (donor) Water acts as both (acceptor) (donor) a hydrogen donor CH 3 COOH + H 2 O H 3 O+ + CH 3 COOand hydrogen acceptor – it acts Base Acid as both an acid (acceptor) (donor) and a base. It is + NH 3 + H 2 O NH 4 + OH AMPHOTERIC H 2 O + CH 3 COO- CH 3 COOH + OHAcid (donor) Base (acceptor)
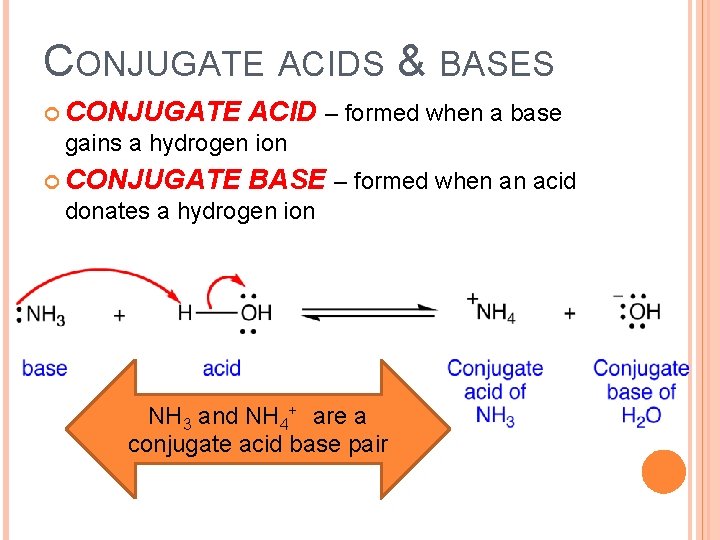
CONJUGATE ACIDS & BASES CONJUGATE ACID – formed when a base gains a hydrogen ion CONJUGATE BASE – formed when an acid donates a hydrogen ion NH 3 and NH 4+ are a conjugate acid base pair
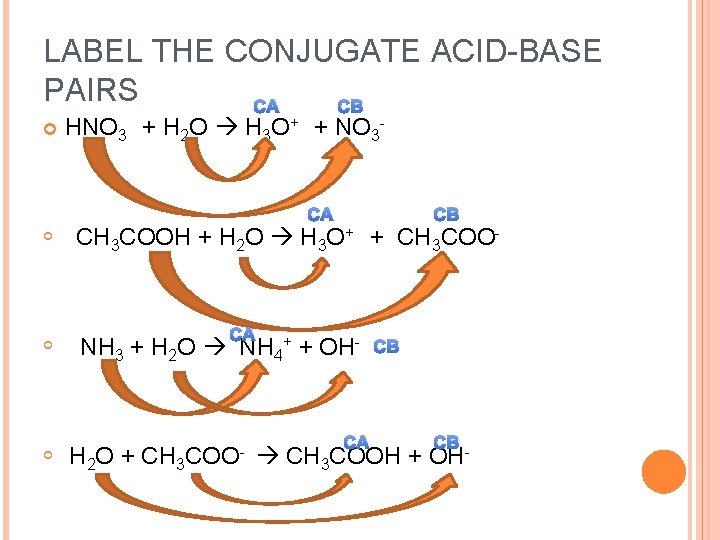
LABEL THE CONJUGATE ACID-BASE PAIRS CA CB HNO 3 + H 2 O H 3 O+ + NO 3 CA CB CH 3 COOH + H 2 O H 3 O+ + CH 3 COO- CA NH 3 + H 2 O NH 4+ + OH- H 2 O + CH 3 COO- CA CB CB CH 3 COOH + OH-
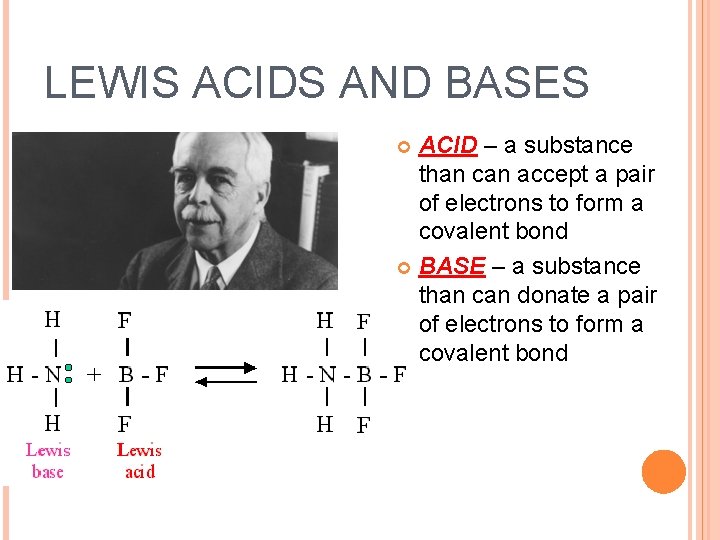
LEWIS ACIDS AND BASES ACID – a substance than can accept a pair of electrons to form a covalent bond BASE – a substance than can donate a pair of electrons to form a covalent bond
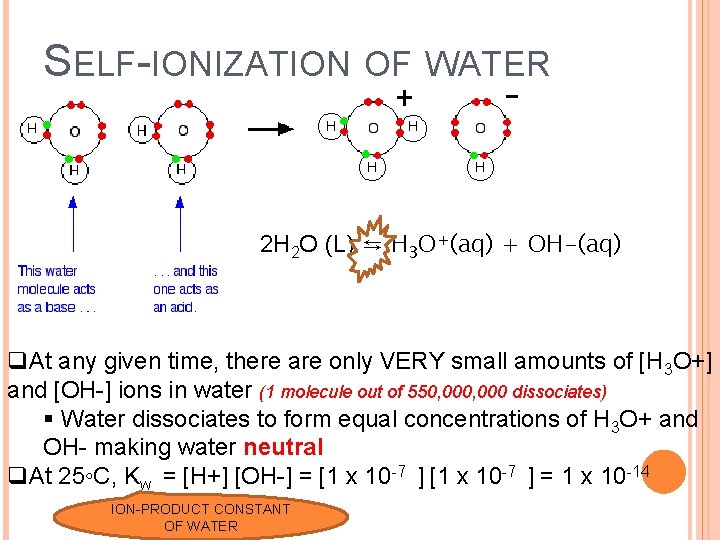
SELF-IONIZATION OF WATER 2 H 2 O (L) ⇆ H 3 O+(aq) + OH-(aq) q. At any given time, there are only VERY small amounts of [H 3 O+] and [OH-] ions in water (1 molecule out of 550, 000 dissociates) § Water dissociates to form equal concentrations of H 3 O+ and OH- making water neutral q. At 25◦C, Kw = [H+] [OH-] = [1 x 10 -7 ] = 1 x 10 -14 ION-PRODUCT CONSTANT OF WATER
![ACIDIC SOLUTIONS VS. BASIC SOLUTIONS Acidic Solution [H+] is greater than [OH-] comes from ACIDIC SOLUTIONS VS. BASIC SOLUTIONS Acidic Solution [H+] is greater than [OH-] comes from](http://slidetodoc.com/presentation_image_h2/b0bb4f77098f0b8a5965228c345fc0e6/image-13.jpg)
ACIDIC SOLUTIONS VS. BASIC SOLUTIONS Acidic Solution [H+] is greater than [OH-] comes from selfionization of water Extra [H+] comes from substances that dissolve in water to create [H+] is greater than 1 x 10 -7 Basic Solution [H+] is less than [OH-] [H+] comes from selfionization of water Extra [OH-] comes from substances that dissolve in water to create [H+] is less than 1 x 10 -7 Also known as alkaline solution
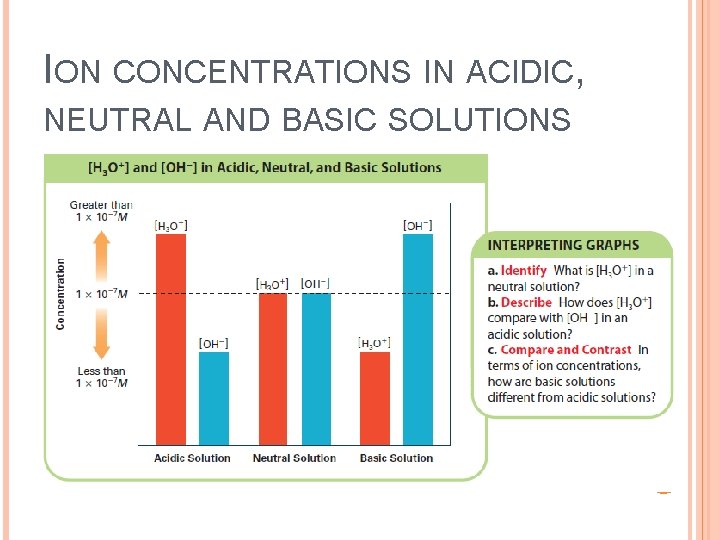
ION CONCENTRATIONS IN ACIDIC, NEUTRAL AND BASIC SOLUTIONS
![ACIDIC, BASIC OR NEUTRAL? [H+] = 6. 0 x 10 -10 M Basic (hydrogen ACIDIC, BASIC OR NEUTRAL? [H+] = 6. 0 x 10 -10 M Basic (hydrogen](http://slidetodoc.com/presentation_image_h2/b0bb4f77098f0b8a5965228c345fc0e6/image-15.jpg)
ACIDIC, BASIC OR NEUTRAL? [H+] = 6. 0 x 10 -10 M Basic (hydrogen ion concentration is less than 1. 0 x 10 -7 [OH-] = 3. 0 x 10 -2 M [OH-][H+]= 1. 0 x 10 -14 3. 0 x 10 -2 [H+] = 3. 3 x 10 -13 Basic [H+] = 2. 0 x 10 -7 M Acidic (hydrogen ion concentration is greater than 1. 0 x 10 -7 [OH-] = 1. 0 x 10 -7 M Neutral
![ACIDIC SOLUTIONS VS. BASIC SOLUTIONS Acidic Solution [H+] is greater than [OH-] comes from ACIDIC SOLUTIONS VS. BASIC SOLUTIONS Acidic Solution [H+] is greater than [OH-] comes from](http://slidetodoc.com/presentation_image_h2/b0bb4f77098f0b8a5965228c345fc0e6/image-16.jpg)
ACIDIC SOLUTIONS VS. BASIC SOLUTIONS Acidic Solution [H+] is greater than [OH-] comes from selfionization of water Extra [H+] comes from substances that dissolve in water to create [H+] is greater than 1 x 10 -7 p. H < 7. 0 p. OH > 7. 0 Basic Solution [H+] is less than [OH-] [H+] comes from selfionization of water Extra [OH-] comes from substances that dissolve in water to create [H+] is less than 1 x 10 -7 Also known as alkaline solution p. H > 7. 0 p. OH < 7. 0
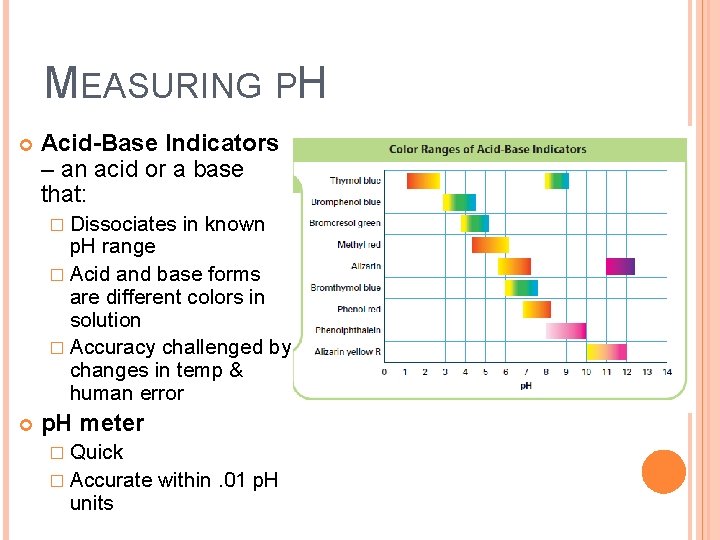
MEASURING PH Acid-Base Indicators – an acid or a base that: � Dissociates in known p. H range � Acid and base forms are different colors in solution � Accuracy challenged by changes in temp & human error p. H meter � Quick � Accurate units within. 01 p. H

ACID RAIN
![PH & POH p. H = –log[H+] p. OH = –log[OH-] p. H + PH & POH p. H = –log[H+] p. OH = –log[OH-] p. H +](http://slidetodoc.com/presentation_image_h2/b0bb4f77098f0b8a5965228c345fc0e6/image-19.jpg)
PH & POH p. H = –log[H+] p. OH = –log[OH-] p. H + p. OH = 14 Use LOG key on calculator not LN to calculate p. H! Use antilog (10 x) key on calculator to calculate M from p. H
![SAMPLE PROBLEMS What is the p. H of a solution if the [H+] is SAMPLE PROBLEMS What is the p. H of a solution if the [H+] is](http://slidetodoc.com/presentation_image_h2/b0bb4f77098f0b8a5965228c345fc0e6/image-20.jpg)
SAMPLE PROBLEMS What is the p. H of a solution if the [H+] is 8 x 10 -11 M? What is the p. OH of a solution if the [OH-] is 2 x 10 -3 M? p. H = -log[H+] p. H = -log[8 x 10 -11] p. H = 10. 10 Some calculators require that you push LOG key first while some require that you put in the # first then press LOG p. OH = -log[OH-] p. OH = -log[2 x 10 -3 ] p. OH = 2. 70 Some calculators may keep giving you errors if you try converting into – log. Just calculate using positive and switch the answer sign
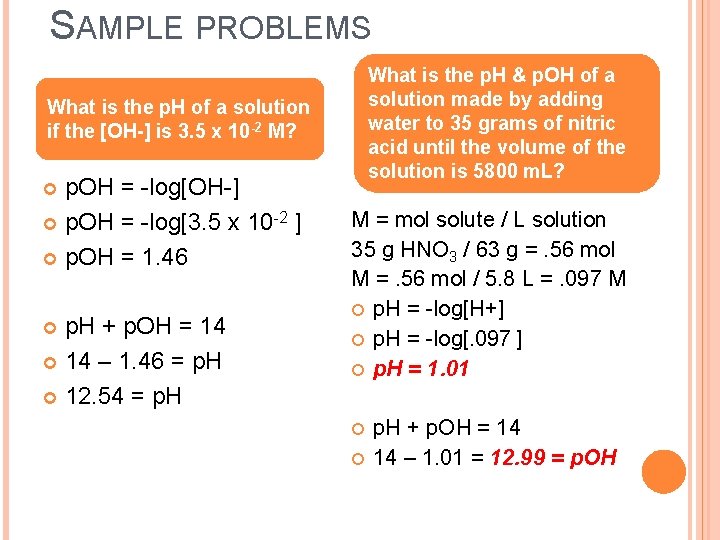
SAMPLE PROBLEMS What is the p. H & p. OH of a solution made by adding water to 35 grams of nitric acid until the volume of the solution is 5800 m. L? What is the p. H of a solution if the [OH-] is 3. 5 x 10 -2 M? p. OH = -log[OH-] p. OH = -log[3. 5 x 10 -2 ] p. OH = 1. 46 p. H + p. OH = 14 – 1. 46 = p. H 12. 54 = p. H M = mol solute / L solution 35 g HNO 3 / 63 g =. 56 mol M =. 56 mol / 5. 8 L =. 097 M p. H = -log[H+] p. H = -log[. 097 ] p. H = 1. 01 p. H + p. OH = 14 14 – 1. 01 = 12. 99 = p. OH
![PLACE THE FOLLOWING COMPOUNDS IN ORDER OF INCREASING [H+] 1 M weak acid 1 PLACE THE FOLLOWING COMPOUNDS IN ORDER OF INCREASING [H+] 1 M weak acid 1](http://slidetodoc.com/presentation_image_h2/b0bb4f77098f0b8a5965228c345fc0e6/image-22.jpg)
PLACE THE FOLLOWING COMPOUNDS IN ORDER OF INCREASING [H+] 1 M weak acid 1 M strong base 1 M weak base 1 M strong base 1 M weak acid 1 M strong acid
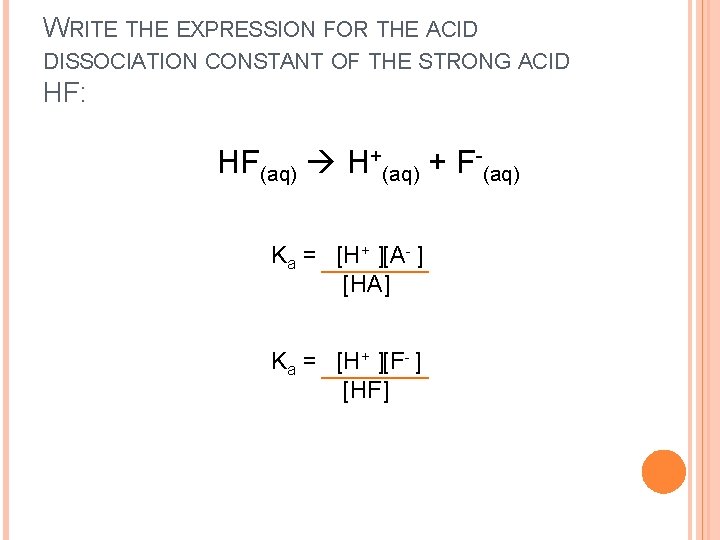
WRITE THE EXPRESSION FOR THE ACID DISSOCIATION CONSTANT OF THE STRONG ACID HF: HF(aq) H+(aq) + F-(aq) Ka = [H+ ][A- ] [HA] Ka = [H+ ][F- ] [HF]
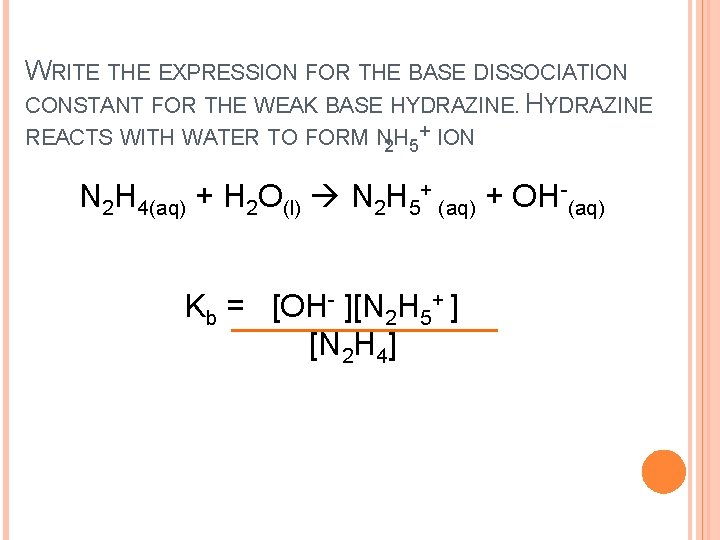
WRITE THE EXPRESSION FOR THE BASE DISSOCIATION CONSTANT FOR THE WEAK BASE HYDRAZINE REACTS WITH WATER TO FORM N 2 H 5+ ION N 2 H 4(aq) + H 2 O(l) N 2 H 5+ (aq) + OH-(aq) Kb = [OH- ][N 2 H 5+ ] [N 2 H 4]
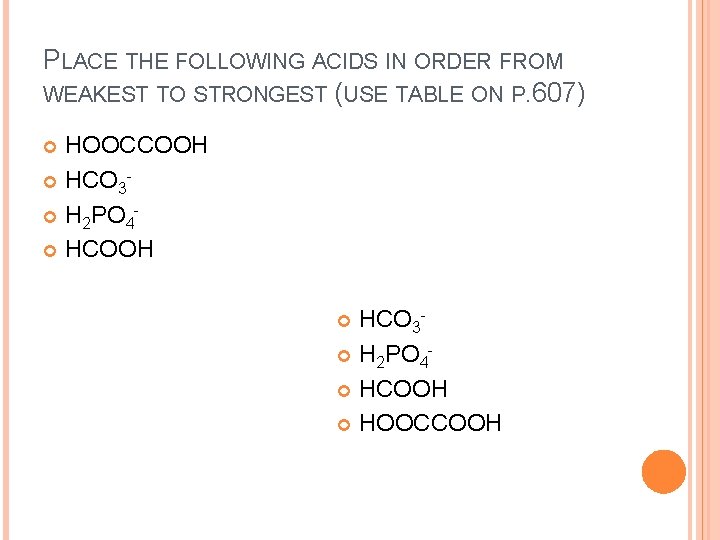
PLACE THE FOLLOWING ACIDS IN ORDER FROM WEAKEST TO STRONGEST (USE TABLE ON P. 607) HOOCCOOH HCO 3 H 2 PO 4 HCOOH HOOCCOOH
![A. 10 M SOLUTION OF FORMIC ACID HAS AN EQUILIBRIUM [H+] = 4. 2 A. 10 M SOLUTION OF FORMIC ACID HAS AN EQUILIBRIUM [H+] = 4. 2](http://slidetodoc.com/presentation_image_h2/b0bb4f77098f0b8a5965228c345fc0e6/image-26.jpg)
A. 10 M SOLUTION OF FORMIC ACID HAS AN EQUILIBRIUM [H+] = 4. 2 X 10 -3 M. WHAT IS THE KA OF FORMIC ACID? HCOOH(aq) H+(aq) + HCOO-(aq) (4. 2 x 10 -3 ) (. 10 – 4. 2 x 10 -3 ) Ka = 1. 8 x 10 -4
- Slides: 26