Acids and Bases 1 What are Acids Acids

Acids and Bases 1

What are Acids? • Acids are common • Some are dangerous and can burn your skin • Some are safe to eat and drink • Stomach acid helps digest food explosion
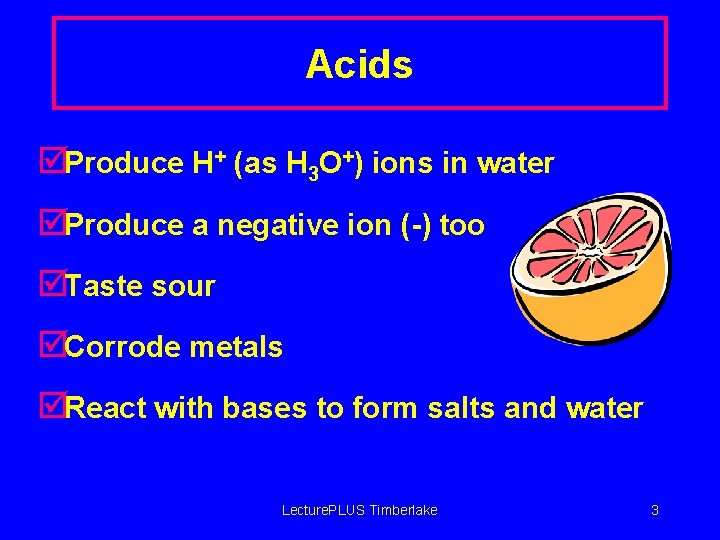
Acids þProduce H+ (as H 3 O+) ions in water þProduce a negative ion (-) too þTaste sour þCorrode metals þReact with bases to form salts and water Lecture. PLUS Timberlake 3

Acids • Definition – – A group of compounds which behave similarly All have low p. H Turn Litmus paper RED All donate H+ ions in aqueous solution • Examples – – Hydrochloric HCl Sulfuric H 2 SO 4 Nitric HNO 3 Ethanoic CH 3 COOH

What are Bases (Alkalis)? • In our home we often use bases to clean things. Eg Bleach and toothpaste • Some things are not acids or bases, we say that they are neutral. Eg Water
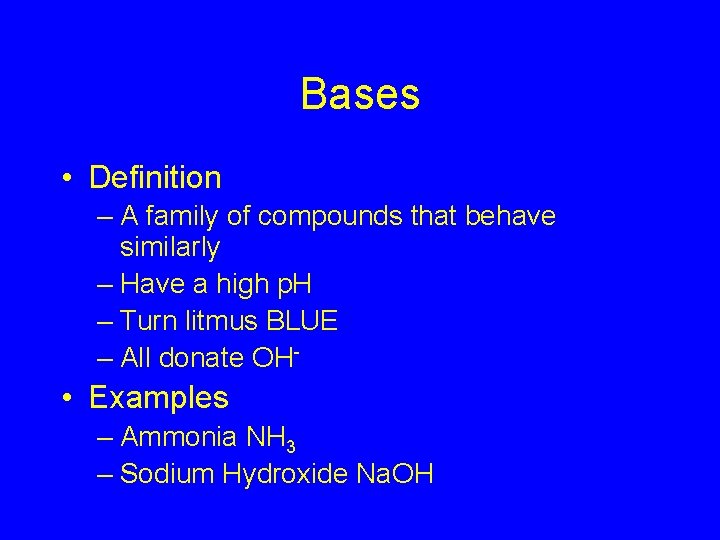
Bases • Definition – A family of compounds that behave similarly – Have a high p. H – Turn litmus BLUE – All donate OH- • Examples – Ammonia NH 3 – Sodium Hydroxide Na. OH

Bases l. Produce OH- ions in water l. Taste bitter, chalky l. Are electrolytes l. Feel soapy, slippery l. React with acids to form salts and water Lecture. PLUS Timberlake 7
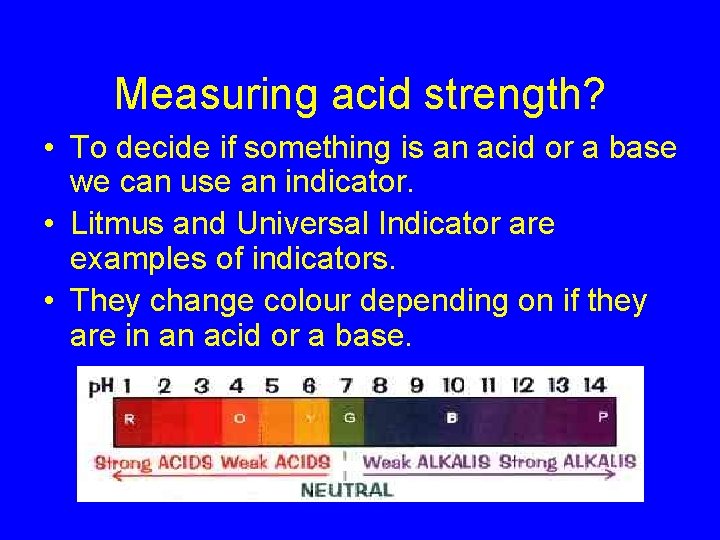
Measuring acid strength? • To decide if something is an acid or a base we can use an indicator. • Litmus and Universal Indicator are examples of indicators. • They change colour depending on if they are in an acid or a base.
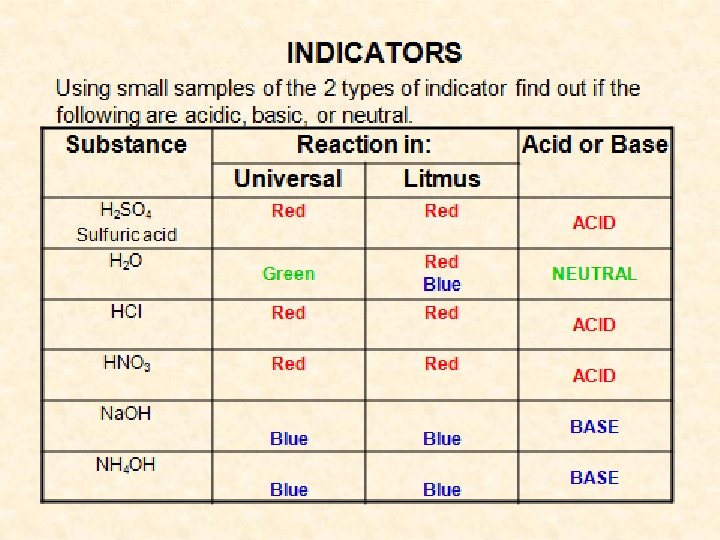
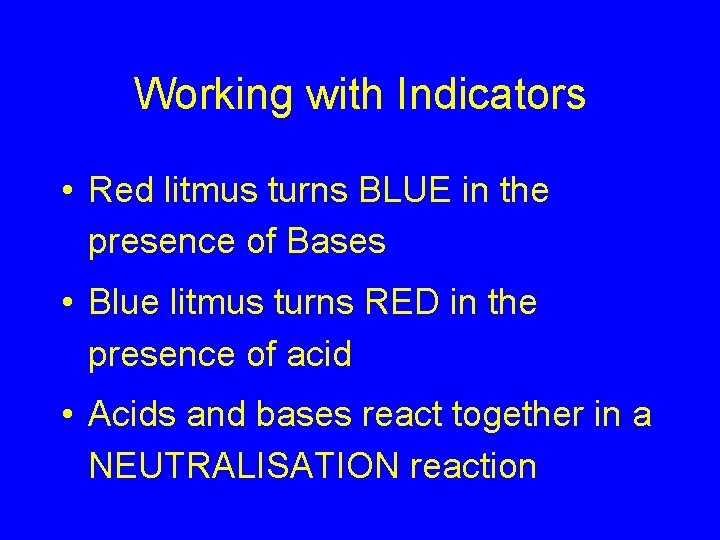
Working with Indicators • Red litmus turns BLUE in the presence of Bases • Blue litmus turns RED in the presence of acid • Acids and bases react together in a NEUTRALISATION reaction

Learning Check AB 1 Describe the solution in each of the following as: 1) acid 2) base or 3)neutral. A. ___soda B. ___soap C. ___coffee D. ___ wine E. ___ water F. ___ grapefruit Lecture. PLUS Timberlake 11

Solution AB 1 Describe each solution as: 1) acid 2) base or 3) neutral. A. _1_ soda B. _2_ soap C. _1_ coffee D. _1_ wine E. _3_ water F. _1_ grapefruit Lecture. PLUS Timberlake 12
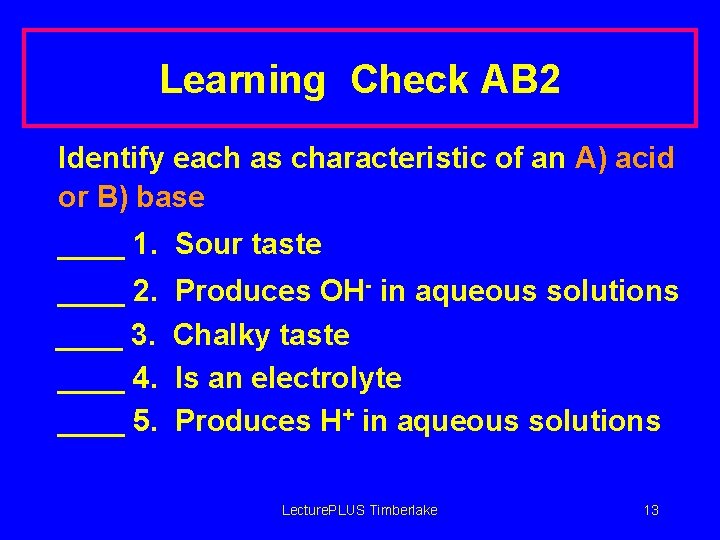
Learning Check AB 2 Identify each as characteristic of an A) acid or B) base ____ 1. Sour taste ____ 2. ____ 3. ____ 4. ____ 5. Produces OH- in aqueous solutions Chalky taste Is an electrolyte Produces H+ in aqueous solutions Lecture. PLUS Timberlake 13

Solution AB 2 Identify each as a characteristic of an A) acid or B) base _A_ 1. Sour taste _B_ 2. Produces OH- in aqueous solutions _B_ 3. Chalky taste A, B 4. Is an electrolyte _A_ 5. Produces H+ in aqueous solutions Lecture. PLUS Timberlake 14

Some Common Acids HCl hydrochloric acid HNO 3 nitric acid H 3 PO 4 phosphoric acid H 2 SO 4 sulfuric acid CH 3 COOH acetic acid Lecture. PLUS Timberlake 15
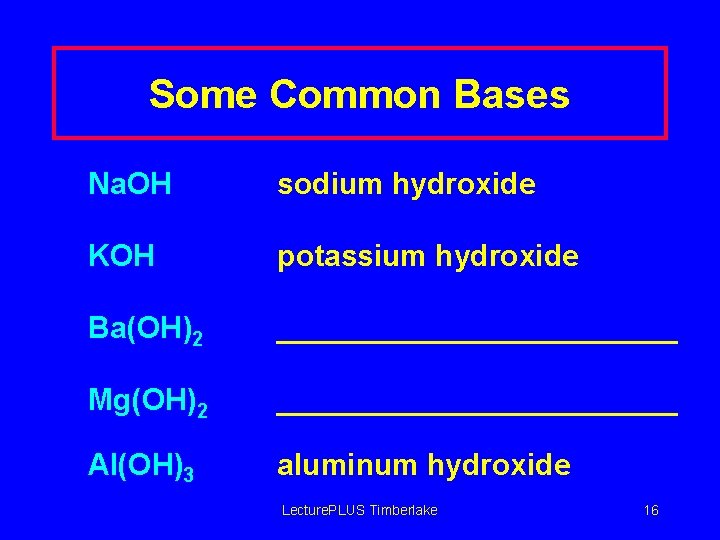
Some Common Bases Na. OH sodium hydroxide KOH potassium hydroxide Ba(OH)2 ____________ Mg(OH)2 ____________ Al(OH)3 aluminum hydroxide Lecture. PLUS Timberlake 16
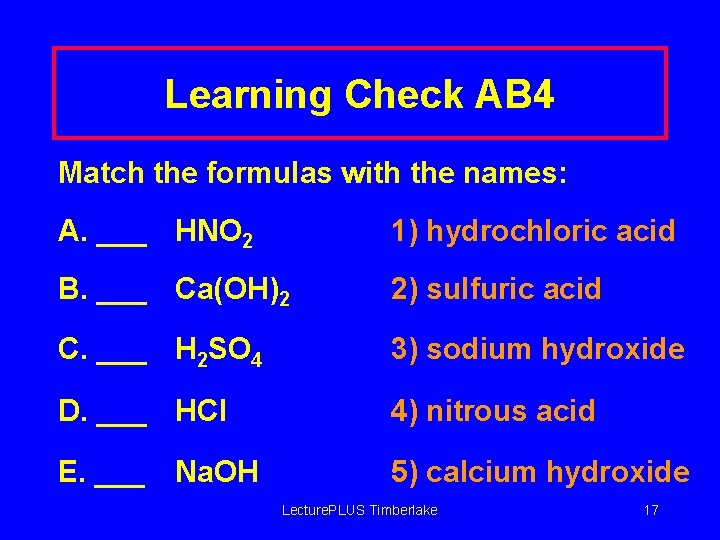
Learning Check AB 4 Match the formulas with the names: A. ___ HNO 2 1) hydrochloric acid B. ___ Ca(OH)2 2) sulfuric acid C. ___ H 2 SO 4 3) sodium hydroxide D. ___ HCl 4) nitrous acid E. ___ 5) calcium hydroxide Na. OH Lecture. PLUS Timberlake 17

Solution AB 4 Match the formulas with the names: A. _4__ HNO 2 1) hydrochloric acid B. _5__ Ca(OH)2 2) sulfuric acid C. _2__ H 2 SO 4 3) sodium hydroxide D. _1__ HCl 4) nitrous acid E. _3__ Na. OH 5) calcium hydroxide Lecture. PLUS Timberlake 18
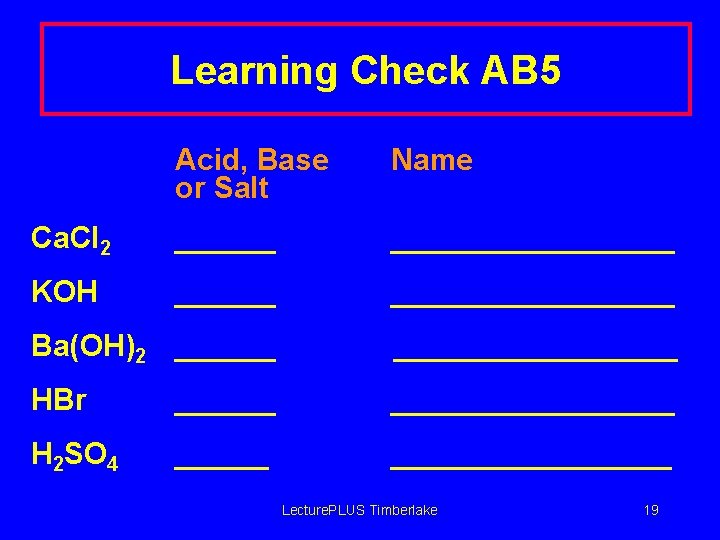
Learning Check AB 5 Acid, Base or Salt Name Ca. Cl 2 ____________ KOH ____________ Ba(OH)2 ____________ HBr ____________ H 2 SO 4 ____________ Lecture. PLUS Timberlake 19

Solution AB 5 Acid, Base or Salt Name Ca. Cl 2 salt calcium chloride KOH base potassiuim hydroxide Ba(OH)2 base barium hydroxide HBr acid hydrobromic acid H 2 SO 4 acid sulfuric acid Lecture. PLUS Timberlake 20

Acids • A dilute acid has lots of water and a small amount of acid • A concentrated acid has lots of acid and not much water so must be handled carefully • A strong acid releases lots of H+ • A weak acid releases fewer H+

Strong and Weak Acids and Bases Strong acids HCl, HNO 3 , H 2 SO 4 Most other acids are weak. Strong bases Na. OH, KOH, and Ca(OH)2 Most other bases are weak. Lecture. PLUS Timberlake 22
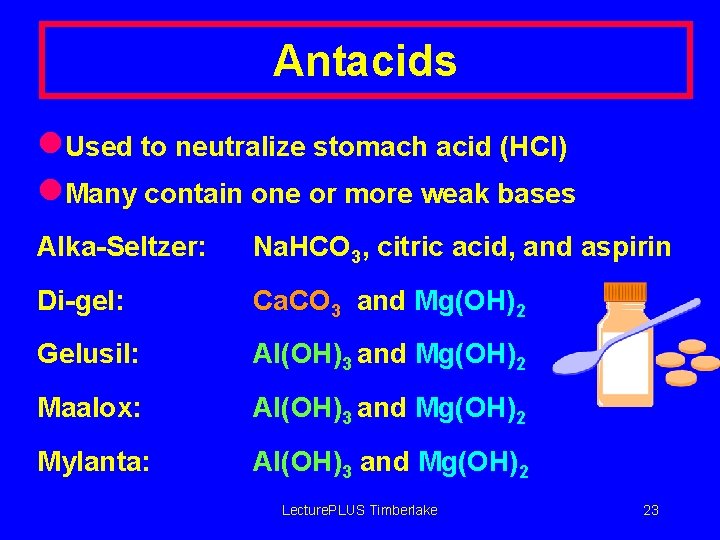
Antacids l. Used to neutralize stomach acid (HCl) l. Many contain one or more weak bases Alka-Seltzer: Na. HCO 3, citric acid, and aspirin Di-gel: Ca. CO 3 and Mg(OH)2 Gelusil: Al(OH)3 and Mg(OH)2 Maalox: Al(OH)3 and Mg(OH)2 Mylanta: Al(OH)3 and Mg(OH)2 Lecture. PLUS Timberlake 23
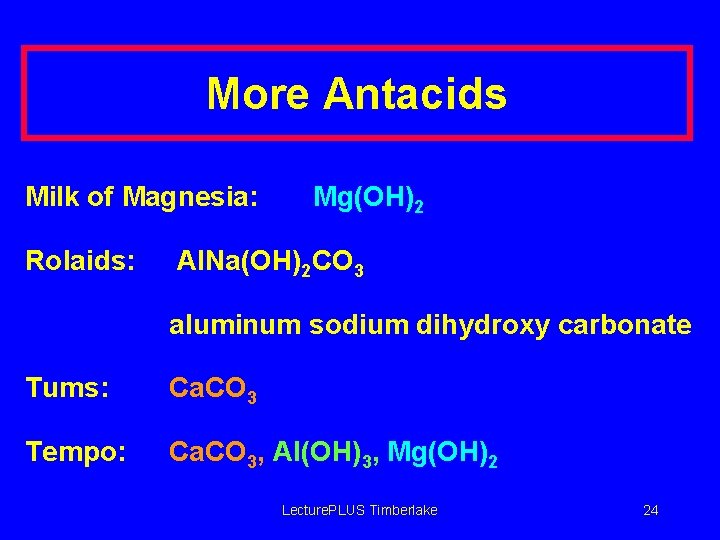
More Antacids Milk of Magnesia: Rolaids: Mg(OH)2 Al. Na(OH)2 CO 3 aluminum sodium dihydroxy carbonate Tums: Ca. CO 3 Tempo: Ca. CO 3, Al(OH)3, Mg(OH)2 Lecture. PLUS Timberlake 24

Dilutions l Add water l Volume increases. l New concentration is less than initial Lecture. PLUS Timberlake 25
![p. H l Indicates the acidity [H 3 O+] of the solution l p. p. H l Indicates the acidity [H 3 O+] of the solution l p.](http://slidetodoc.com/presentation_image_h2/7bc2bec9f89130d1e62ce03b89022492/image-26.jpg)
p. H l Indicates the acidity [H 3 O+] of the solution l p. H = - log [H 3 O+] l From the French pouvoir hydrogene (“hydrogen power” or power of hydrogen) Lecture. PLUS Timberlake 26
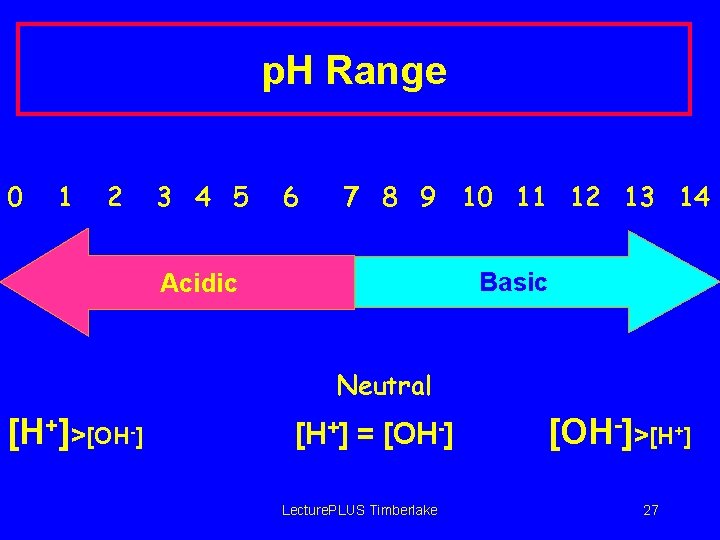
p. H Range 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Basic Acidic Neutral [H+]>[OH-] [H+] = [OH-] Lecture. PLUS Timberlake [OH-]>[H+] 27
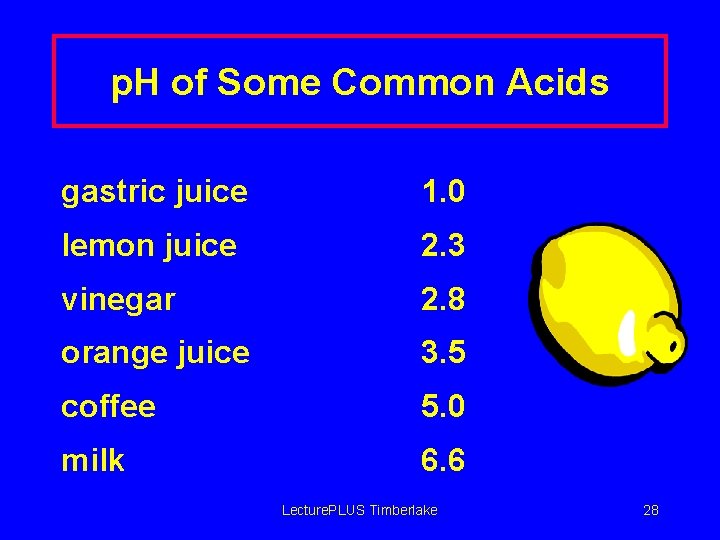
p. H of Some Common Acids gastric juice 1. 0 lemon juice 2. 3 vinegar 2. 8 orange juice 3. 5 coffee 5. 0 milk 6. 6 Lecture. PLUS Timberlake 28

p. H of Some Common Bases blood 7. 4 tears 7. 4 seawater 8. 4 milk of magnesia 10. 6 household ammonia 11. 0 Lecture. PLUS Timberlake 29

Acid Rain • Consider – Causes • natural • human – Effects • • buildings flora fauna health – Chemical equations – Prevention/Solution

Acid Rain l. Unpolluted rain has a p. H of 5. 6 l. Rain with a p. H below 5. 6 is “acid rain“ l. CO 2 in the air forms carbonic acid CO 2 + H 2 O H 2 CO 3 l. Adds to H+ of rain H 2 CO 3 H+ (aq) + HCO 3 -(aq) Lecture. PLUS Timberlake 31

Sources of Acid Rain l. Power stations l. Oil refineries l. Coal with high S content l. Car and truck emissions l. Bacterial decomposition, and lighting hitting N 2 Lecture. PLUS Timberlake 32

SO 2 NO and NO 2 26 million tons in 1980 22 million tons in 1980 Mt. St Helens (1980) 400, 000 tons SO 2 l Reactions with oxygen in air form SO 3 2 SO 2 + O 2 2 SO 3 l Reactions with water in air form acids SO 3 + H 2 O H 2 SO 4 sulfuric acid NO + H 2 O HNO 2 nitrous acid HNO 2 + H 2 O HNO 3 nitric acid Lecture. PLUS Timberlake 33

Effects of Acid Rain l Leaches Al from soil, which kills fish l Fish kills in spring from runoff due to accumulation of large amounts of acid in snow l Dissolves waxy coatings that protect leaves from bacteria l Corrodes metals, textiles, paper and leather Lecture. PLUS Timberlake 34
- Slides: 34