AcidBase Titrations Introduction to Acids and Bases Chapter

Acid-Base Titrations

Introduction to Acids and Bases Chapter 8
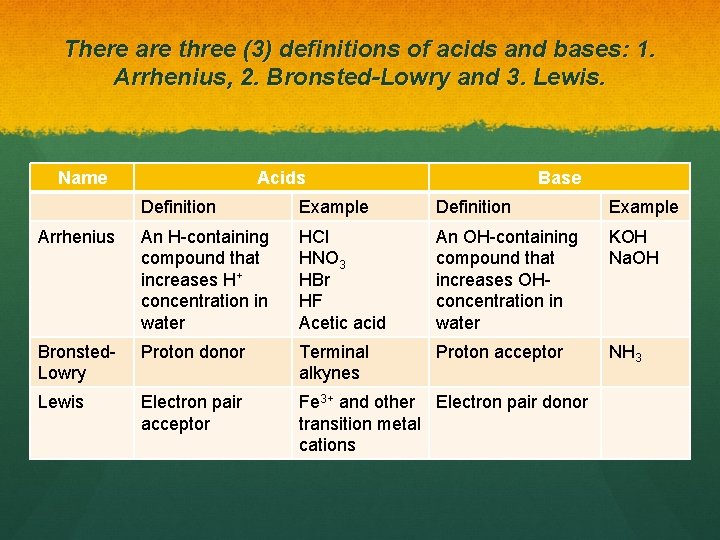
There are three (3) definitions of acids and bases: 1. Arrhenius, 2. Bronsted-Lowry and 3. Lewis. Name Acids Base Definition Example Arrhenius An H-containing compound that increases H+ concentration in water HCl HNO 3 HBr HF Acetic acid An OH-containing compound that increases OHconcentration in water KOH Na. OH Bronsted. Lowry Proton donor Terminal alkynes Proton acceptor NH 3 Lewis Electron pair acceptor Fe 3+ and other Electron pair donor transition metal cations
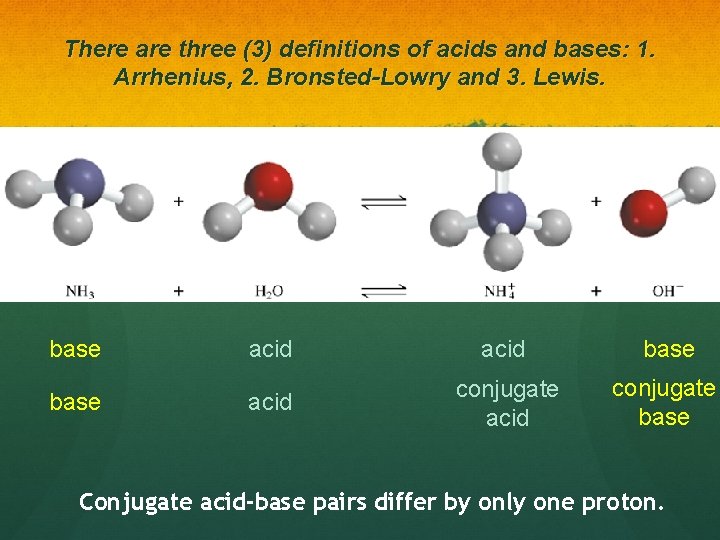
There are three (3) definitions of acids and bases: 1. Arrhenius, 2. Bronsted-Lowry and 3. Lewis. base acid conjugate base Conjugate acid-base pairs differ by only one proton.
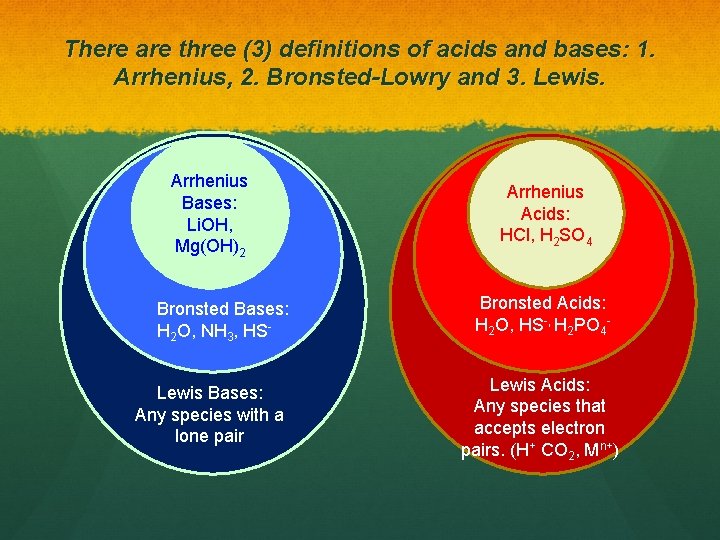
There are three (3) definitions of acids and bases: 1. Arrhenius, 2. Bronsted-Lowry and 3. Lewis. Arrhenius Bases: Li. OH, Mg(OH)2 Bronsted Bases: H 2 O, NH 3, HSLewis Bases: Any species with a lone pair Arrhenius Acids: HCl, H 2 SO 4 Bronsted Acids: H 2 O, HS-, H 2 PO 4 Lewis Acids: Any species that accepts electron pairs. (H+ CO 2, Mn+)
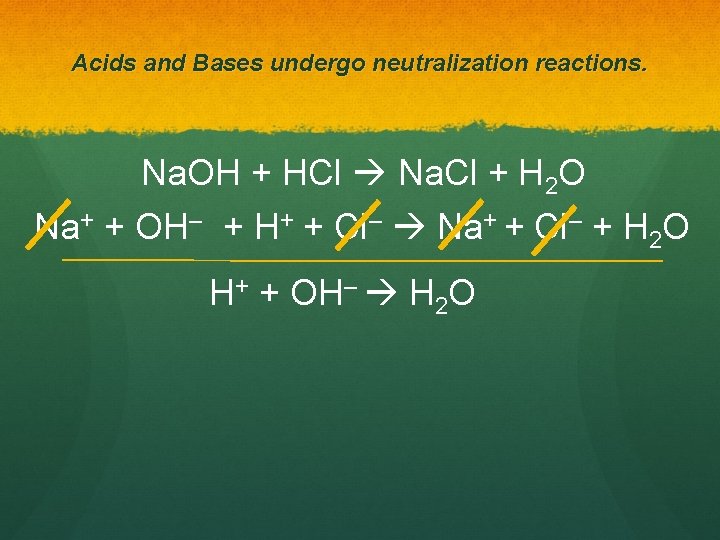
Acids and Bases undergo neutralization reactions. Na. OH + HCl Na. Cl + H 2 O Na+ + OH– + H+ + Cl– Na+ + Cl– + H 2 O H+ + OH– H 2 O
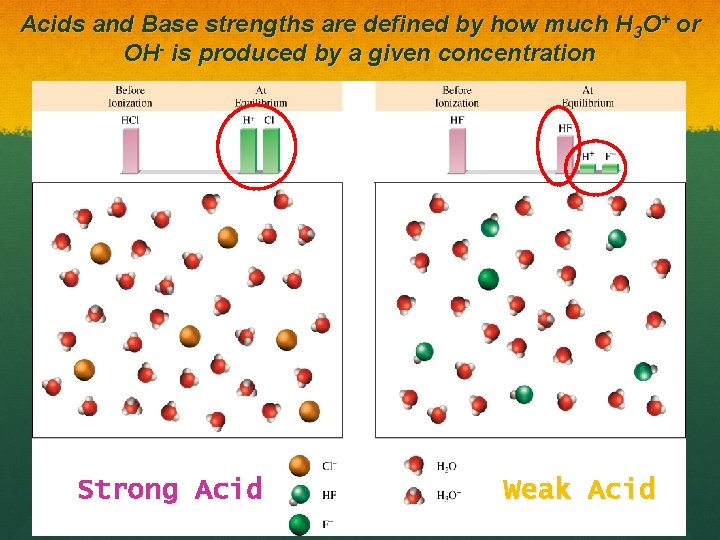
Acids and Base strengths are defined by how much H 3 O+ or OH- is produced by a given concentration Strong Acid Weak Acid
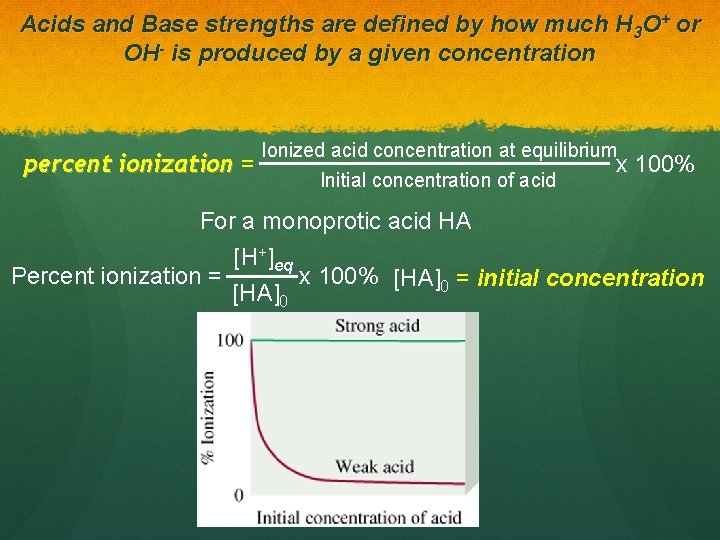
Acids and Base strengths are defined by how much H 3 O+ or OH- is produced by a given concentration percent ionization = Ionized acid concentration at equilibrium x Initial concentration of acid 100% For a monoprotic acid HA Percent ionization = [H+]eq [HA]0 x 100% [HA]0 = initial concentration
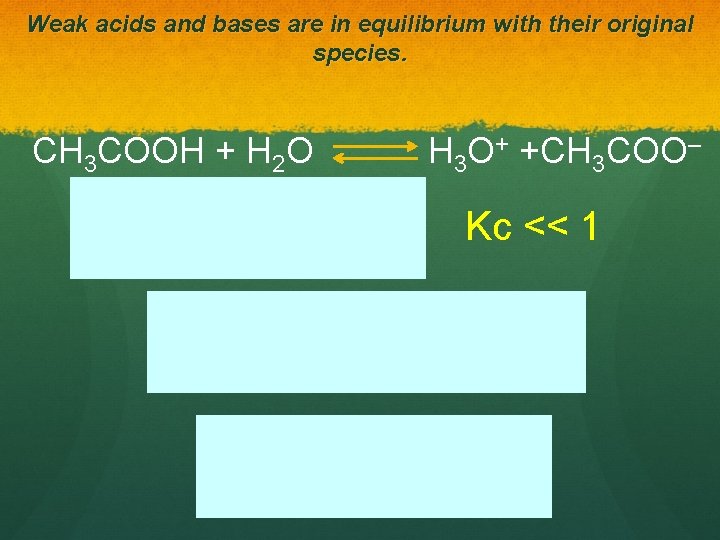
Weak acids and bases are in equilibrium with their original species. CH 3 COOH + H 2 O H 3 O+ +CH 3 COO– Kc << 1
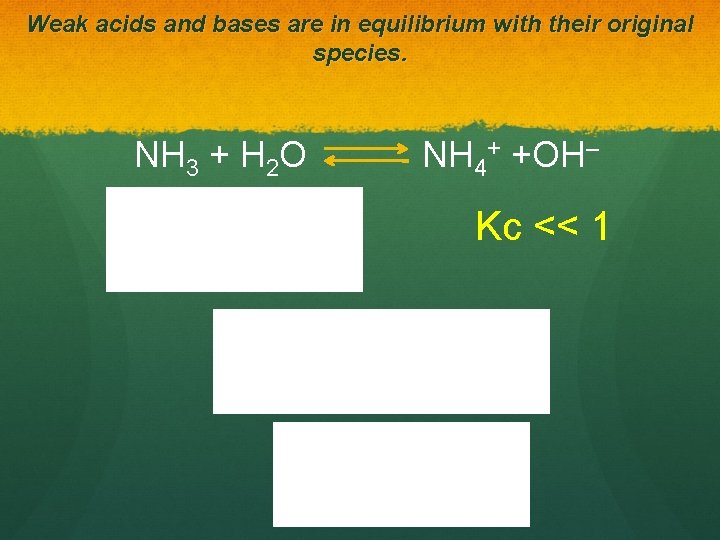
Weak acids and bases are in equilibrium with their original species. NH 3 + H 2 O NH 4+ +OH– Kc << 1
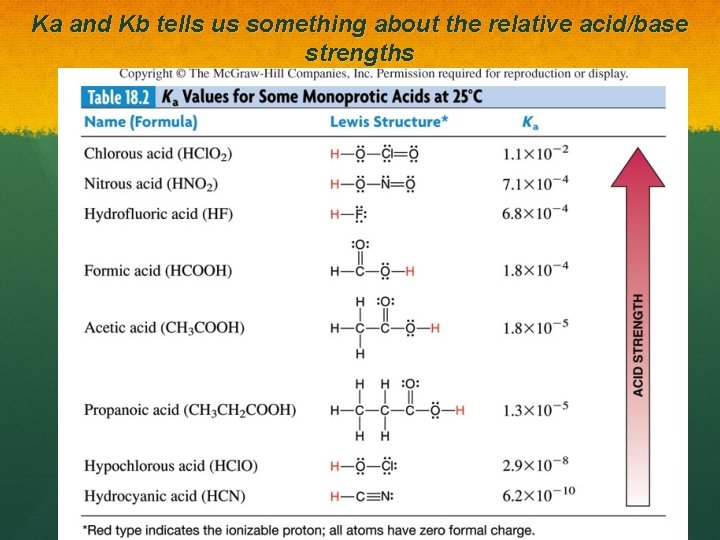
Ka and Kb tells us something about the relative acid/base strengths
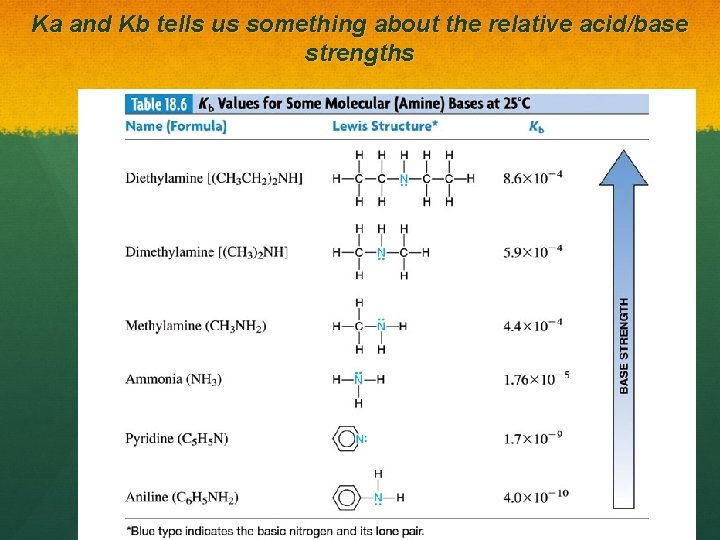
Ka and Kb tells us something about the relative acid/base strengths
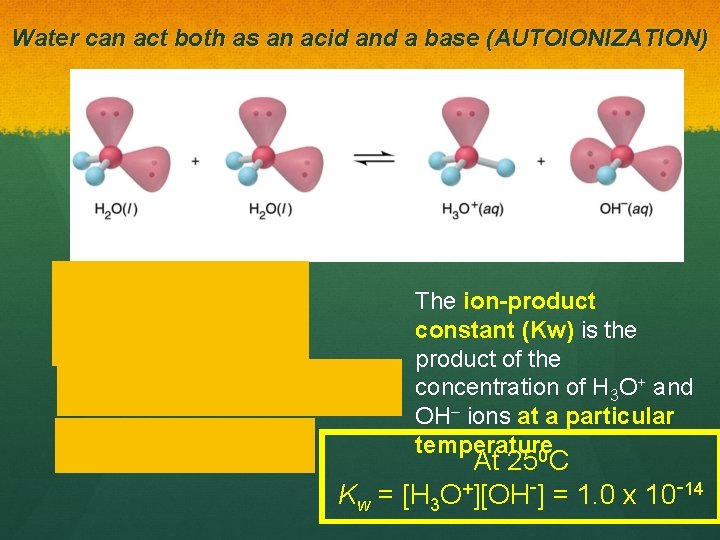
Water can act both as an acid and a base (AUTOIONIZATION) The ion-product constant (Kw) is the product of the concentration of H 3 O+ and OH– ions at a particular temperature 0 At 25 C Kw = [H 3 O+][OH-] = 1. 0 x 10 -14
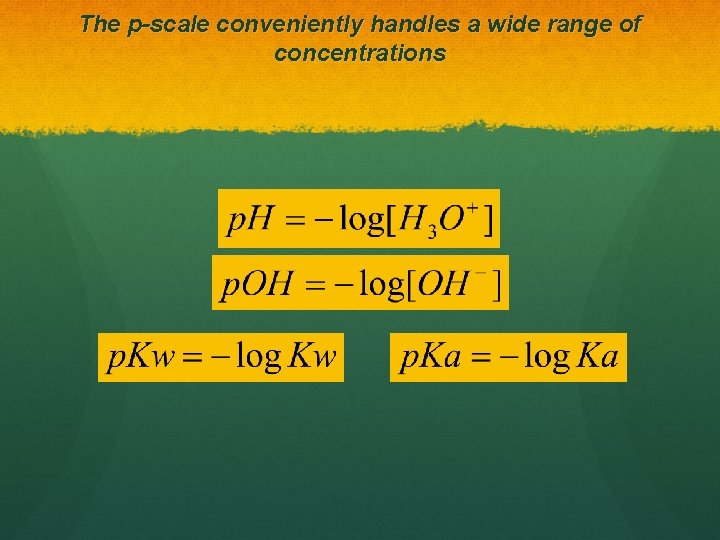
The p-scale conveniently handles a wide range of concentrations

Solving weak acid ionization problems: 1. Identify the major species that can affect the p. H. • In most cases, you can ignore the autoionization of water. [H 3 O+] from water is negligible in comparison to [H 3 O+] from the weak acid. • Ignore [OH-] because it is determined by [H+]. 2. Use ICE to express the equilibrium concentrations in terms of single unknown x. 3. Write Ka in terms of equilibrium concentrations. Solve for x by the approximation method. If approximation is not valid, solve for x exactly. 4. Calculate concentrations of all species and/or p. H of the solution.

Exercises What is the p. H of a 0. 5 M HF solution (at 25°C, Ka = 7. 1 x 10 -4)? What is the p. H of a 0. 05 M HF solution? What is the p. H of a 0. 122 M monoprotic acid whose Ka is 5. 7 x 10 -4?
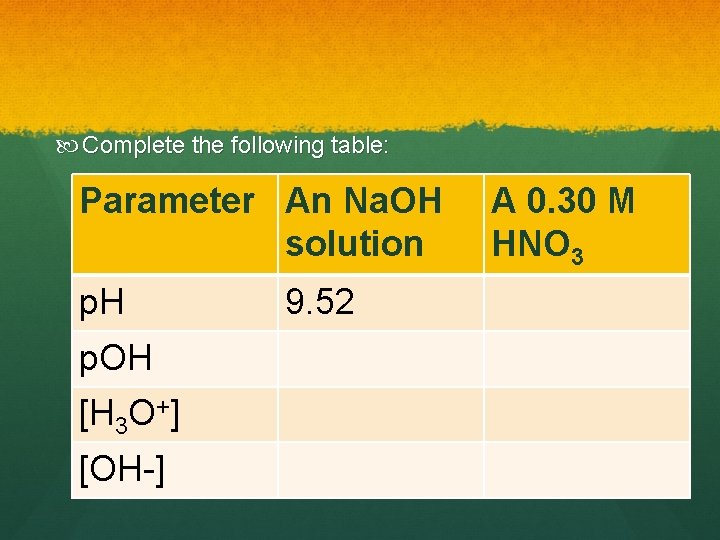
Complete the following table: Parameter An Na. OH solution p. H p. OH [H 3 O+] [OH-] 9. 52 A 0. 30 M HNO 3
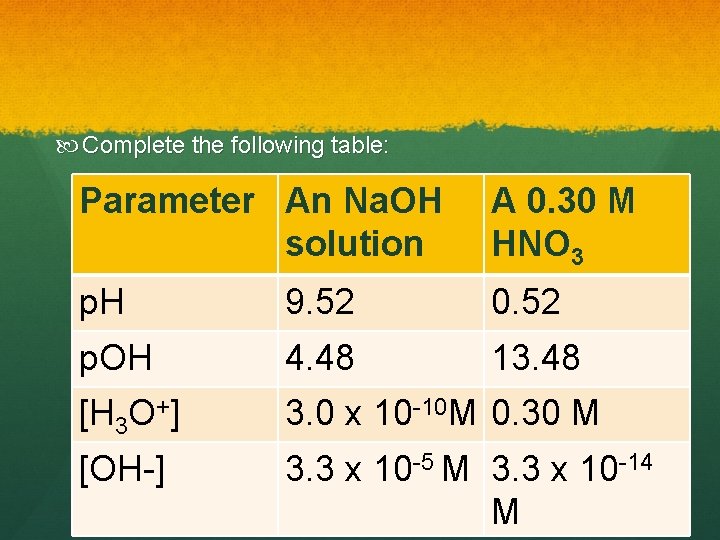
Complete the following table: Parameter An Na. OH solution A 0. 30 M HNO 3 p. H 9. 52 0. 52 p. OH 4. 48 13. 48 [H 3 O+] 3. 0 x 10 -10 M 0. 30 M [OH-] 3. 3 x 10 -5 M 3. 3 x 10 -14 M
![Exercises What is the p. H, [H 3 O+], [OH-] of 7. 52 x Exercises What is the p. H, [H 3 O+], [OH-] of 7. 52 x](http://slidetodoc.com/presentation_image_h2/3d16c56a7c56c0c3acd616ae0c29886c/image-19.jpg)
Exercises What is the p. H, [H 3 O+], [OH-] of 7. 52 x 10 -4 M Cs. OH? What is the p. OH, [H 3 O+], [OH-] of 1. 59 x 10 -3 M HCl. O 4? What is the [H 3 O+], [OH-] and p. OH in a solution with a p. H of 2. 77 What is the [H 3 O+], [OH-] and p. H in a solution with a p. OH of 11. 27

Exercises Chloroacetic acid has a p. Ka of 2. 87. What are [H 3 O+], p. H, [Cl. CH 2 COOH], [Cl. CH 2 COO-] in 1. 05 M [Cl. CH 2 COOH] A 0. 735 M of weak acid is 12. 5% dissociated. Calculate [H 3 O+], p. H, [OH-], p. OH of solution. Calculate Ka of acid

Buffers Chapter 9

Buffers solutions are solutions that resist changes in p. H

Buffers contain appreciable amounts of a weak acid and its conjugate base HA/ A– NH 4 Cl/NH 3 H 3 PO 4/Na. H 2 PO 4 NH 4 SH/Na 2 S HCOOH/HCOO K HBr/KBr H 3 IO 3/Li 2 HIO 3 Na. OH/Na 2 O

Buffers contain appreciable amounts of a weak acid and its conjugate base What do we mean by o A 50 -m. L solution of 0. 25 M Acetic acid. appreciable? o A 50 -m. L solution of 0. 25 M Sodium Acetate o A solution containing 0. 125 M Acetic acid and 0. 125 M Acetate HA / A– + base + acid ** If we take the ratio of base to acid or acid to base, it should be within 10% of each other
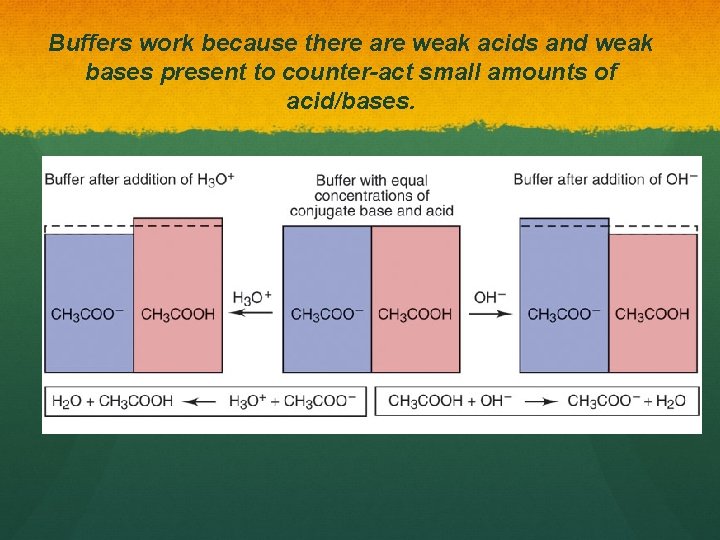
Buffers work because there are weak acids and weak bases present to counter-act small amounts of acid/bases.
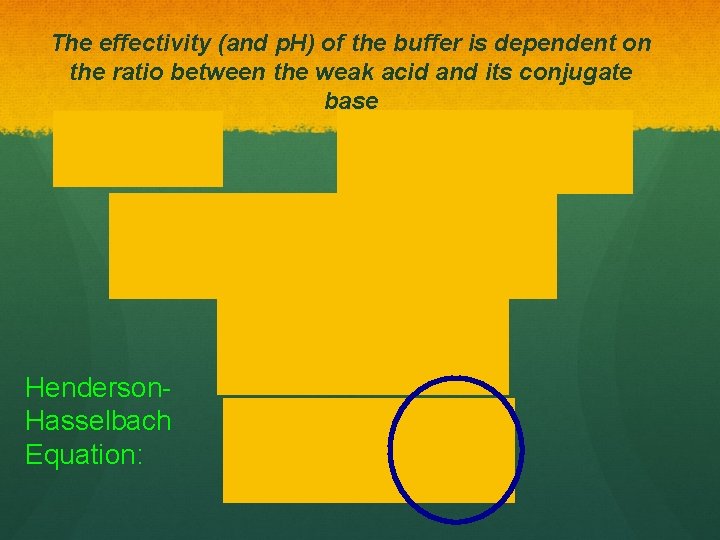
The effectivity (and p. H) of the buffer is dependent on the ratio between the weak acid and its conjugate base Henderson. Hasselbach Equation:
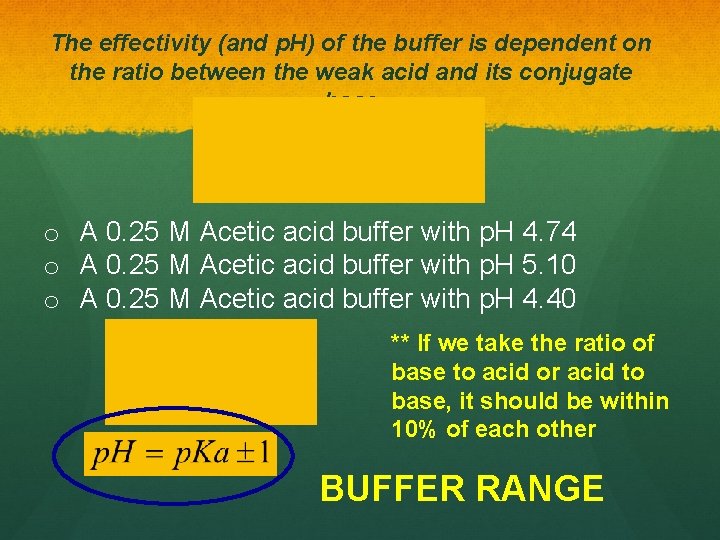
The effectivity (and p. H) of the buffer is dependent on the ratio between the weak acid and its conjugate base o A 0. 25 M Acetic acid buffer with p. H 4. 74 o A 0. 25 M Acetic acid buffer with p. H 5. 10 o A 0. 25 M Acetic acid buffer with p. H 4. 40 ** If we take the ratio of base to acid or acid to base, it should be within 10% of each other BUFFER RANGE
The effectivity (and p. H) of the buffer is dependent on the ratio between the weak acid and its conjugate base o A buffer for p. H 10. 00 o A buffer for p. H 4. 00 o A buffer for p. H 7. 00 The closer the p. H of the buffer to the p. Ka the better
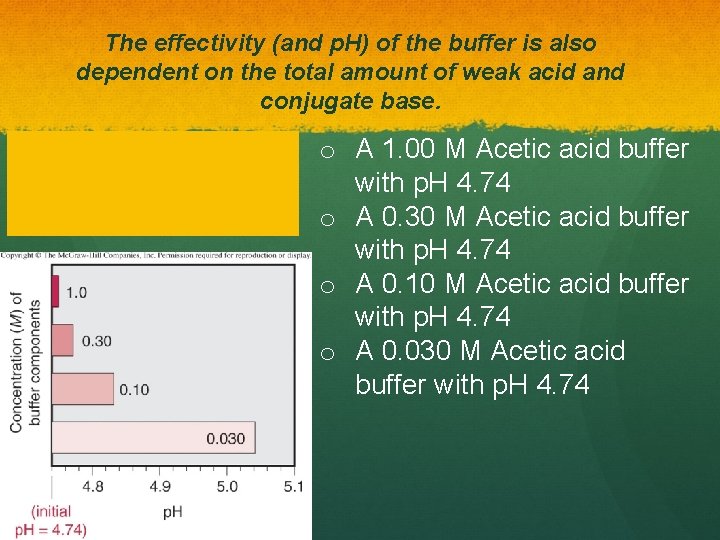
The effectivity (and p. H) of the buffer is also dependent on the total amount of weak acid and conjugate base. o A 1. 00 M Acetic acid buffer with p. H 4. 74 o A 0. 30 M Acetic acid buffer with p. H 4. 74 o A 0. 10 M Acetic acid buffer with p. H 4. 74 o A 0. 030 M Acetic acid buffer with p. H 4. 74
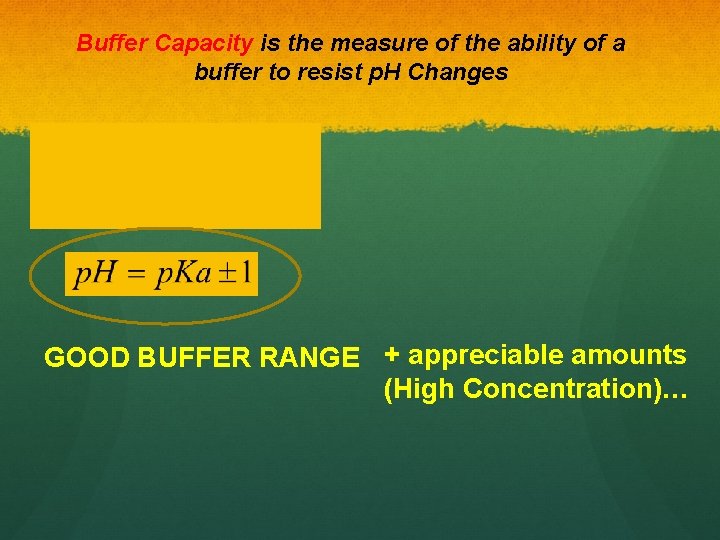
Buffer Capacity is the measure of the ability of a buffer to resist p. H Changes GOOD BUFFER RANGE + appreciable amounts (High Concentration)…
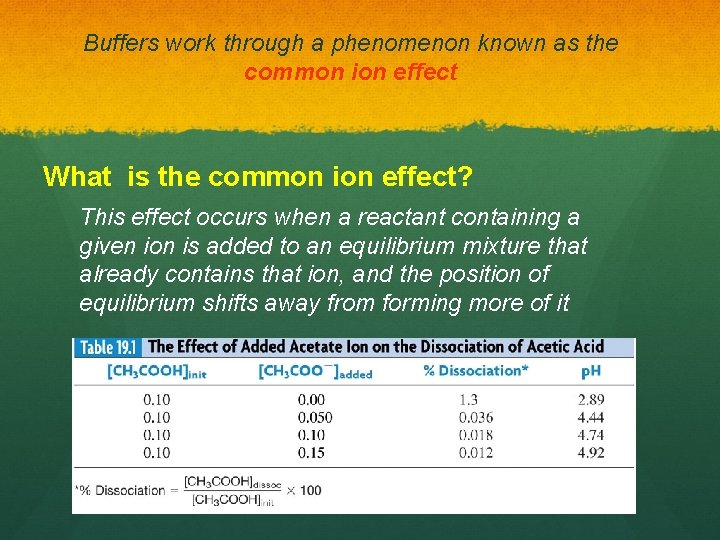
Buffers work through a phenomenon known as the common ion effect What is the common ion effect? This effect occurs when a reactant containing a given ion is added to an equilibrium mixture that already contains that ion, and the position of equilibrium shifts away from forming more of it

Preparation of Buffers 1. Choose the conjugate acid-base pair 2. Calculate the ratio of the buffer component concentrations 3. Determine the buffer concentration 4. Mix the solution and adjust p. H

Buffers can be prepared using a weak acid and the salt of its conjugate base (or a weak base and the salt of its conjugate acid). Example 1 Preparing a p. H 10. 00 carbonate buffer. How many grams of Na 2 CO 3 must one add to 1. 5 L of freshly prepared 0. 20 M Na. HCO 3 to make the buffer? Ka of HCO 3 - is 4. 7 x 10 -11 Example 2 Prepare a 50 -m. L of 0. 12 M Acetic acid buffer with equal concentrations of acetic acid and acetate from 3. 00 M acetic acid stock solution and sodium acetate salt

Buffers can also be prepared by using a weak acid (or weak base) then add a strong base (or acid) to desired p. H. 5. 0 g of CH 3 COONa is dissolved in 100. m. L of water. How many m. L of 0. 50 MHCl should be added to form a buffer with p. H 4. 90? Will diluting the final mixture to 500 m. L affect the p. H of the buffer? What is affected by dilution?

Acid-Base Titrations Chapter 10
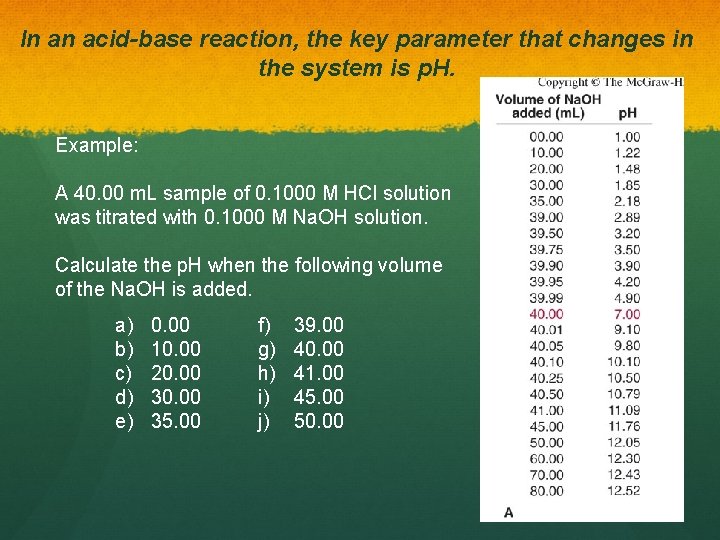
In an acid-base reaction, the key parameter that changes in the system is p. H. Example: A 40. 00 m. L sample of 0. 1000 M HCl solution was titrated with 0. 1000 M Na. OH solution. Calculate the p. H when the following volume of the Na. OH is added. a) b) c) d) e) 0. 00 10. 00 20. 00 35. 00 f) g) h) i) j) 39. 00 40. 00 41. 00 45. 00 50. 00
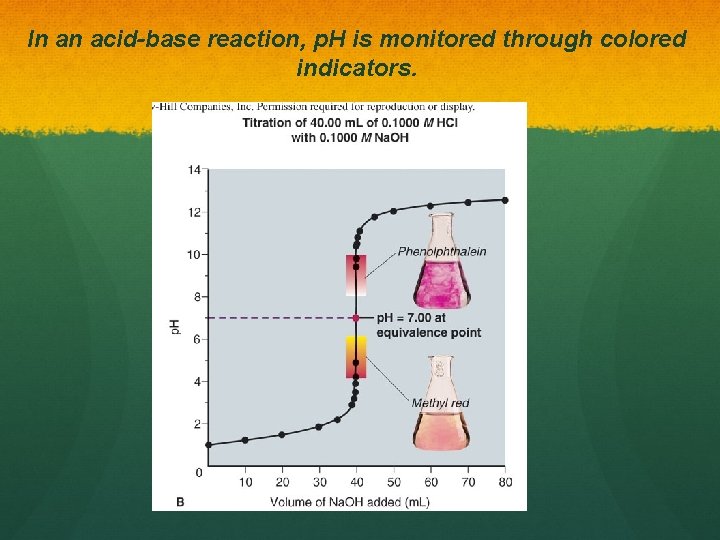
In an acid-base reaction, p. H is monitored through colored indicators.
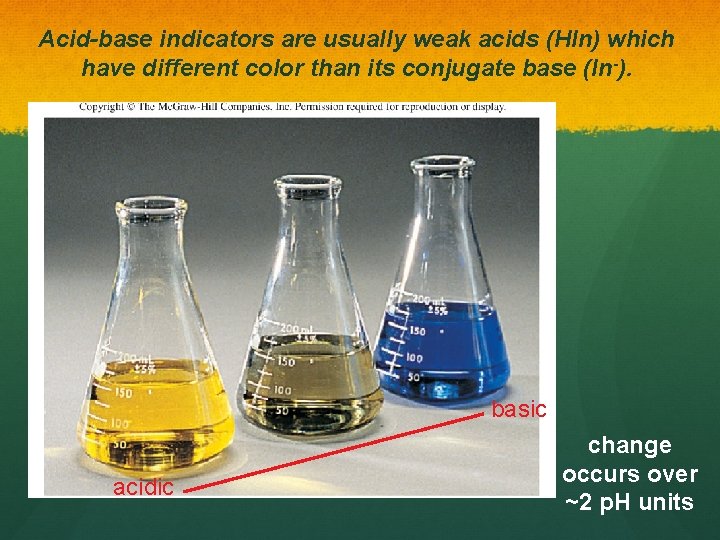
Acid-base indicators are usually weak acids (HIn) which have different color than its conjugate base (In-). basic acidic change occurs over ~2 p. H units
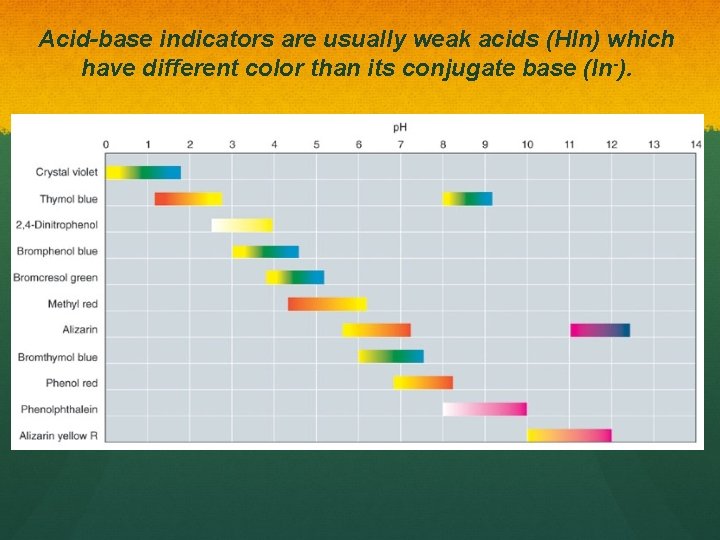
Acid-base indicators are usually weak acids (HIn) which have different color than its conjugate base (In-).
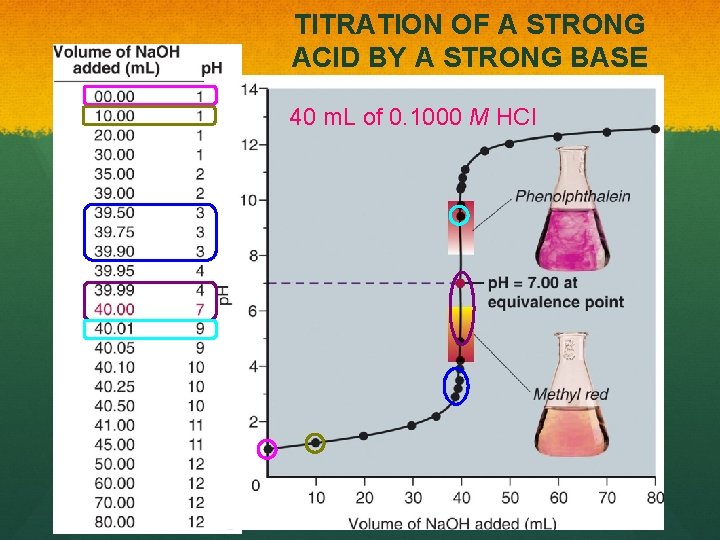
TITRATION OF A STRONG ACID BY A STRONG BASE 40 m. L of 0. 1000 M HCl
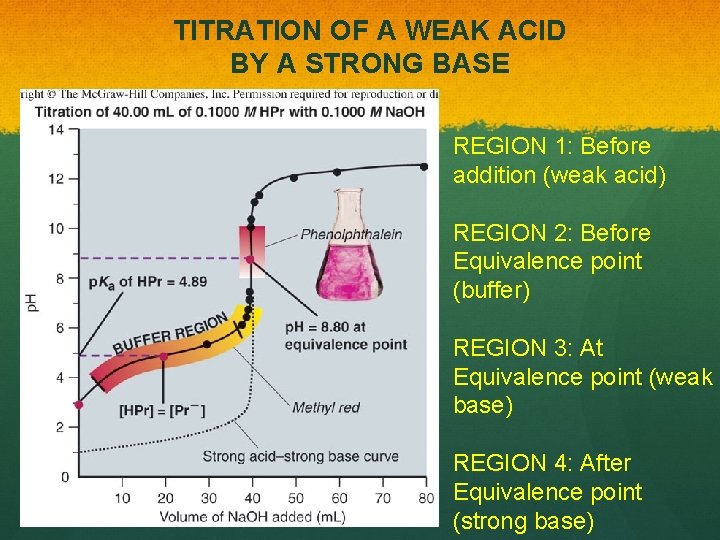
TITRATION OF A WEAK ACID BY A STRONG BASE REGION 1: Before addition (weak acid) REGION 2: Before Equivalence point (buffer) REGION 3: At Equivalence point (weak base) REGION 4: After Equivalence point (strong base)

TITRATION OF A WEAK ACID BY A STRONG BASE EXAMPLE in Book: Titration of 50. 00 m. L of 0. 0200 M MES (p. Ka = 6. 27) with 0. 1000 M Na. OH REGION 1: Before addition (weak acid) REGION 2: Before Equivalence point (buffer) REGION 3: At Equivalence point (weak base) REGION 4: After Equivalence point (strong base)
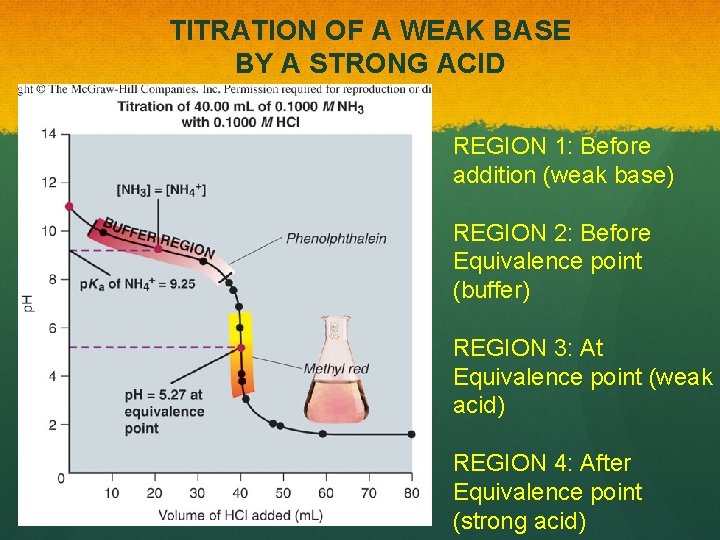
TITRATION OF A WEAK BASE BY A STRONG ACID REGION 1: Before addition (weak base) REGION 2: Before Equivalence point (buffer) REGION 3: At Equivalence point (weak acid) REGION 4: After Equivalence point (strong acid)

TITRATION OF A WEAK BASE BY A STRONG ACID Example in Book: Titration of 25. 00 m. L of 0. 08364 M pyridine (Kb = 1. 6 x 10 -9) with 0. 1067 M HCl. REGION 1: Before addition (weak base) REGION 2: Before Equivalence point (buffer) REGION 3: At Equivalence point (weak acid) REGION 4: After Equivalence point (strong acid)
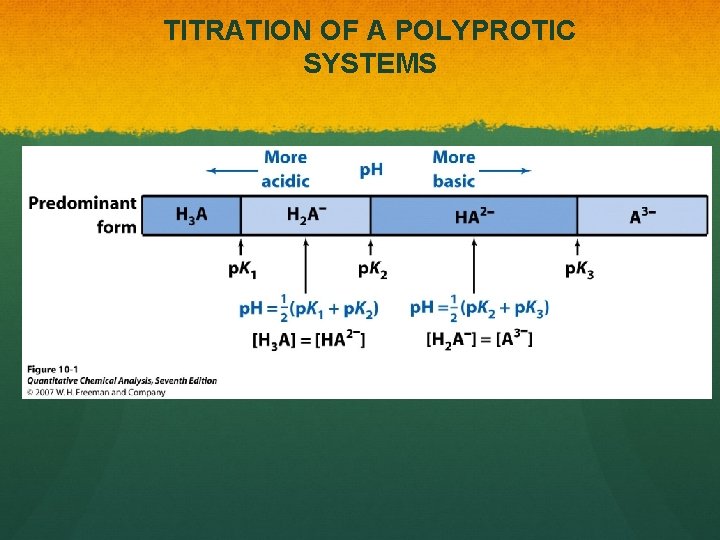
TITRATION OF A POLYPROTIC SYSTEMS
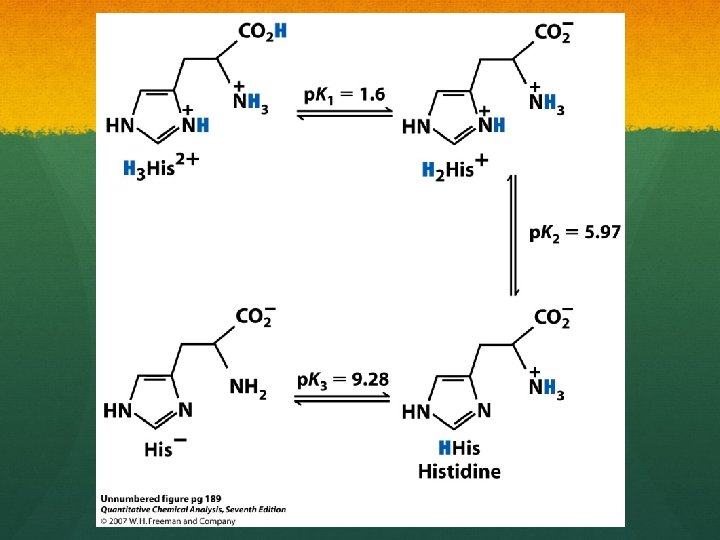
TITRATION OF A POLYPROTIC SYSTEMS
- Slides: 46