AcidBase Titration Buffers Buffers A mixture composed of

Acid/Base Titration Buffers
Buffers A mixture composed of a weak acid and its conjugate base (acidic buffer) OR weak base and its conjugate acid (basic buffer) Allow small amounts of acids and bases into a solution without a huge change in the solution’s p. H Buffer capacity—trait of buffers indicating how much strong acid/base can be tolerated before a solution’s p. H changes drastically.
Why are buffers important to us? Buffers present in our body keep fluids within a certain p. H range. Blood has a p. H range of approximately 7. 3 -7. 4. A buffer involving H 2 CO 3/HCO 3 - maintains the blood p. H

Titration Used to determine UNKNOWN concentrations of solutions through a solution of KNOWN concentration. An acidic or basic solution of KNOWN concentration is added to an acidic/basic solution of UNKNOWN concentration. Indicators such as phenolphtalein display a change when all of the acid or base has been neutralized.

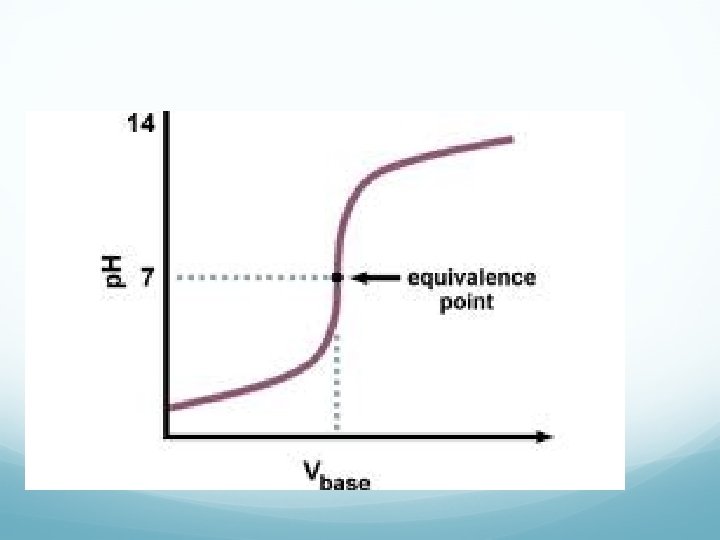
Titration Terminology End Point: point in a titration where a change is observed due to indicator. Equivalence Point: point in a titration where enough base or acid is present to neutralize the acidic or basic solution. We can plot a titration on a graph—titration curve
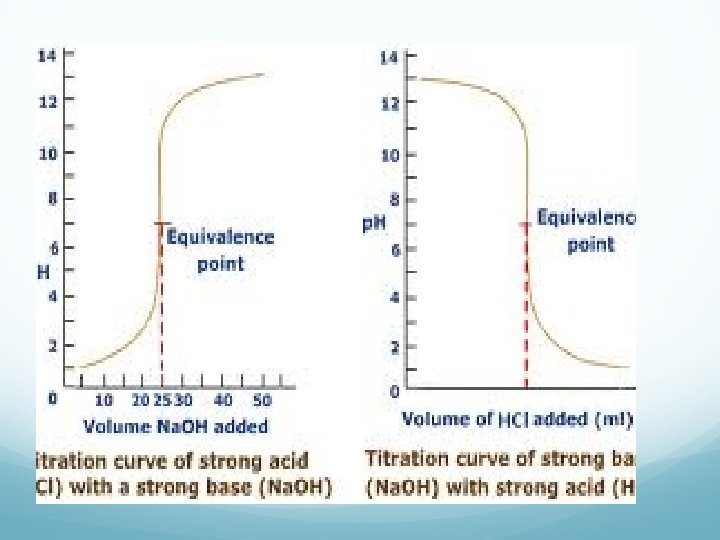
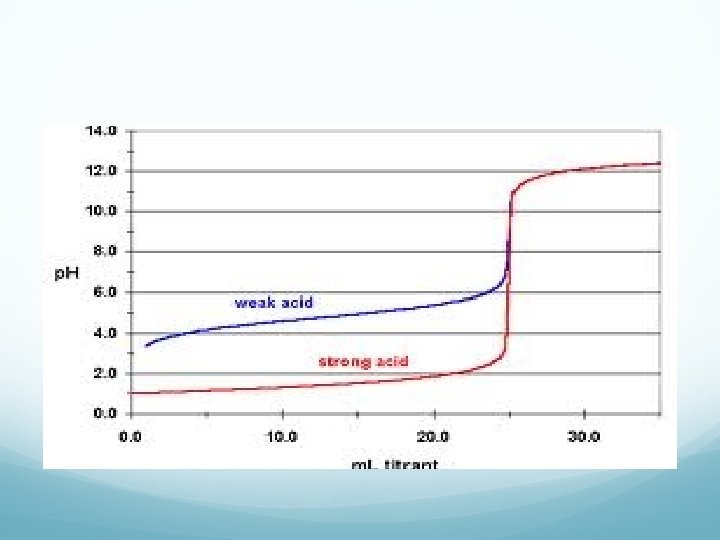

Calculations Ma V a = Mb V b Where M is the molarity & V is the volume Used to determine final concentration vs original concentration. Solve like you would dilution problems.

Example 1: If 20. 0 ml of 0. 0100 M aqueous HCl is required to neutralize 30. 0 ml of an aqueous solution of Na. OH, determine the molarity of the Na. OH solution.

Example 2 A 15. 5 ml sample of 0. 215 M KOH solution required 21. 2 ml of aqueous acetic acid solution in a titration experiment. Calculate the molarity of the acetic acid (CH 3 COOH) solution.

Example 3: If 29. 96 ml of a solution of Ba(OH)2 requires 16. 08 ml of a 2. 303 M solution of HNO 3 for complete titration, what is the molarity of the Ba(OH)2 solution?

Homework Titration Problem Set # 1
- Slides: 14