AcidBase Reactions SNC 2 D Neutralization The reaction

Acid-Base Reactions SNC 2 D

Neutralization The reaction between an acid and a base produces water (H 2 O) and an ionic compound known as a ? ? , which may be dissolved in the water or form as a precipitate.

Neutralization The reaction between an acid and a base produces water (H 2 O) and an ionic compound known as a salt, which may be dissolved in the water or form as a precipitate.

Neutralization The reaction between an acid and a base produces water (H 2 O) and an ionic compound known as a salt, which may be dissolved in the water or form as a precipitate. Example hydrochloric acid + sodium hydroxide

Neutralization The reaction between an acid and a base produces water (H 2 O) and an ionic compound known as a salt, which may be dissolved in the water or form as a precipitate. Example hydrochloric acid + sodium hydroxide water + sodium chloride

Neutralization The reaction between an acid and a base produces water (H 2 O) and an ionic compound known as a salt, which may be dissolved in the water or form as a precipitate. Example hydrochloric acid + sodium hydroxide water + sodium chloride HCl(aq) + Na. OH(aq) H 2 O(l) + Na. Cl(aq)

Neutralization This ________ reaction is also known as a ______ because the products of the reaction are neutral (i. e. have a p. H of ? ).

Neutralization This double displacement reaction is also known as a ______ because the products of the reaction are neutral (i. e. have a p. H of ? ).

Neutralization This double displacement reaction is also known as a neutralization because the products of the reaction are neutral (i. e. have a p. H of ? ).

Neutralization This double displacement reaction is also known as a neutralization because the products of the reaction are neutral (i. e. have a p. H of 7).

Neutralization This double displacement reaction is also known as a neutralization because the products of the reaction are neutral (i. e. have a p. H of 7). Another Example sulphuric acid + potassium hydroxide

Neutralization This double displacement reaction is also known as a neutralization because the products of the reaction are neutral (i. e. have a p. H of 7). Another Example sulphuric acid + potassium hydroxide water + potassium sulphate
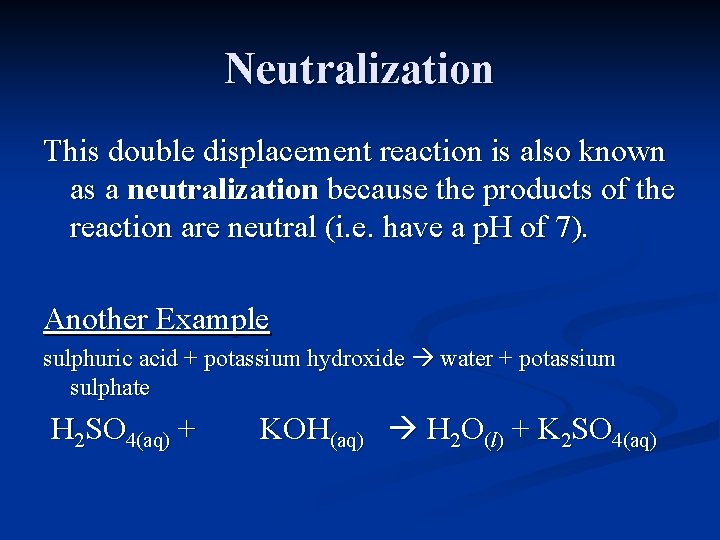
Neutralization This double displacement reaction is also known as a neutralization because the products of the reaction are neutral (i. e. have a p. H of 7). Another Example sulphuric acid + potassium hydroxide water + potassium sulphate H 2 SO 4(aq) + KOH(aq) H 2 O(l) + K 2 SO 4(aq)
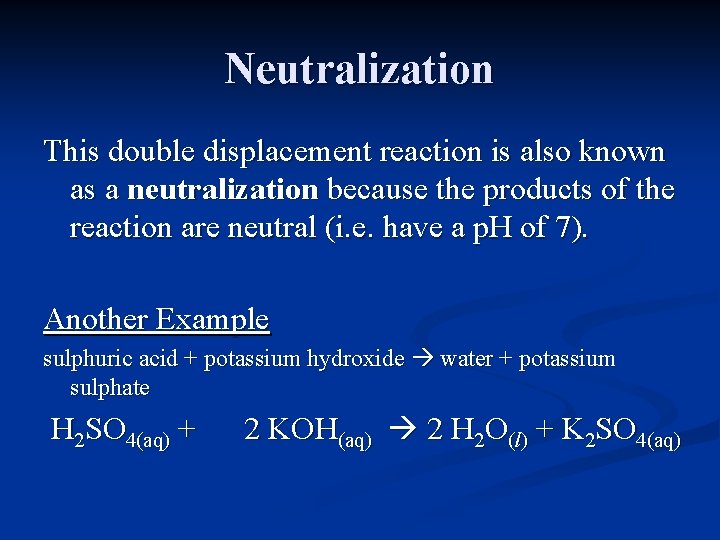
Neutralization This double displacement reaction is also known as a neutralization because the products of the reaction are neutral (i. e. have a p. H of 7). Another Example sulphuric acid + potassium hydroxide water + potassium sulphate H 2 SO 4(aq) + 2 KOH(aq) 2 H 2 O(l) + K 2 SO 4(aq)

Neutralization Some neutralization reactions will also produce carbon dioxide. Example hydrochloric acid + sodium bicarbonate water + sodium chloride + carbon dioxide HCl + Na. HCO 3 H 2 O + Na. Cl + CO 2

Uses Neutralization reactions are useful on small scales, e. g. the formic acid (HCOOH) in a beesting that attacks nerves in the skin can be neutralized with an ammonia-based cream

Uses and on large scales, e. g. lime may be added to an acidified lake to raise its p. H: HNO 3 + Ca(OH)2

Uses and on large scales, e. g. lime may be added to an acidified lake to raise its p. H: HNO 3 + Ca(OH)2 Ca(NO 3)2 + H 2 O

Uses and on large scales, e. g. lime may be added to an acidified lake to raise its p. H: 2 HNO 3 + Ca(OH)2 Ca(NO 3)2 + 2 H 2 O

Activity: Antacids and Neutralization Reactions (refer to handout)
- Slides: 20