AcidBase Reactions An acid is a substance that

Acid-Base Reactions An acid is a substance that produces H+ ions when dissolved in H 2 O. HX H 2 O → H+ (aq) + X- (aq) A base is a substance that produces OH- ions when dissolved in H 2 O. MOH H 2 O → M+ (aq) + OH- (aq) An acid-base reaction is also called a neutralization reaction. 4 -1
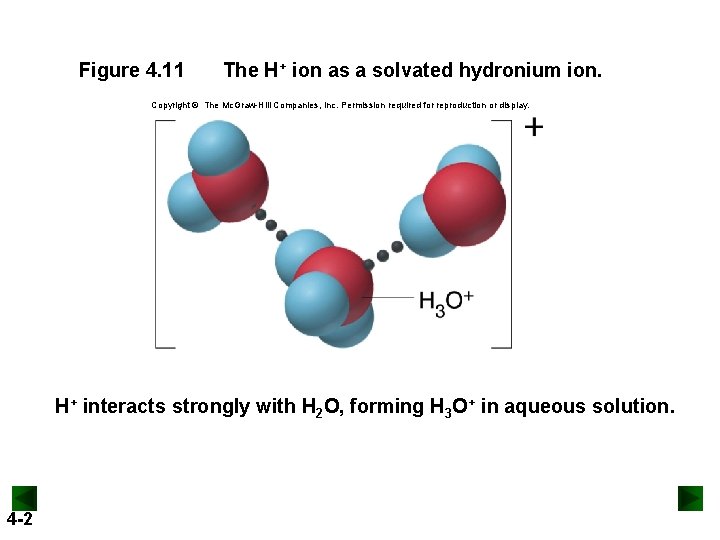
Figure 4. 11 The H+ ion as a solvated hydronium ion. Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display. H+ interacts strongly with H 2 O, forming H 3 O+ in aqueous solution. 4 -2

4 -3

Figure 4. 12 Acids and bases as electrolytes. Strong acids and strong bases dissociate completely into ions in aqueous solution. They are strong electrolytes and conduct well in solution. 4 -4

Figure 4. 12 Acids and bases as electrolytes. Weak acids and weak bases dissociate very little into ions in aqueous solution. They are weak electrolytes and conduct poorly in solution. 4 -5

Sample Problem 4. 12 Determining the Number of H+ (or OH-) Ions in Solution PROBLEM: How many H+(aq) ions are in 25. 3 m. L of 1. 4 M nitric acid? 4 -6

Sample Problem 4. 13 Writing Ionic Equations for Acid-Base Reactions PROBLEM: Write balanced molecular, total ionic, and net ionic equations for the following acid-base reactions and identify the spectator ions. (a) hydrochloric acid (aq) + potassium hydroxide (aq) → (b) strontium hydroxide (aq) + perchloric acid (aq) → (c) barium hydroxide (aq) + sulfuric acid (aq) → 4 -7

4 -8

Figure 4. 13 4 -9 An aqueous strong acid-strong base reaction as a proton-transfer process.
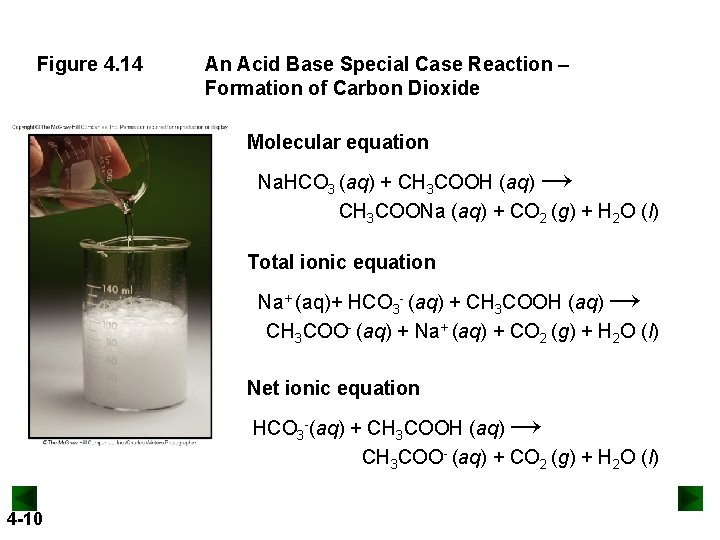
Figure 4. 14 An Acid Base Special Case Reaction – Formation of Carbon Dioxide Molecular equation → Na. HCO 3 (aq) + CH 3 COOH (aq) CH 3 COONa (aq) + CO 2 (g) + H 2 O (l) Total ionic equation → Na+ (aq)+ HCO 3 - (aq) + CH 3 COOH (aq) CH 3 COO- (aq) + Na+ (aq) + CO 2 (g) + H 2 O (l) Net ionic equation → HCO 3 -(aq) + CH 3 COOH (aq) CH 3 COO- (aq) + CO 2 (g) + H 2 O (l) 4 -10

Sample Problem 4. 14 Writing Proton-Transfer Equations for Acid-Base Reactions PROBLEM: Write balanced total and net ionic equations for the following reactions and use curved arrows to show the proton transfer occurs. (a) hydriodic acid (aq) + calcium hydroxide (aq) → Give the name and formula of the salt present when the water evaporates. (b) potassium hydroxide (aq) + propionic acid (aq) → Note that propionic acid is a weak acid. Be sure to identify the spectator ions in this reaction. 4 -11

4 -12
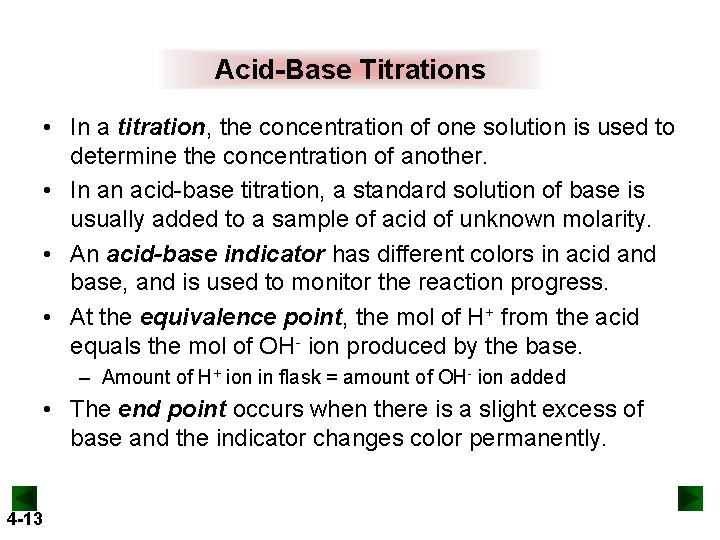
Acid-Base Titrations • In a titration, the concentration of one solution is used to determine the concentration of another. • In an acid-base titration, a standard solution of base is usually added to a sample of acid of unknown molarity. • An acid-base indicator has different colors in acid and base, and is used to monitor the reaction progress. • At the equivalence point, the mol of H+ from the acid equals the mol of OH- ion produced by the base. – Amount of H+ ion in flask = amount of OH- ion added • The end point occurs when there is a slight excess of base and the indicator changes color permanently. 4 -13

Figure 4. 15 4 -14 An acid-base titration.
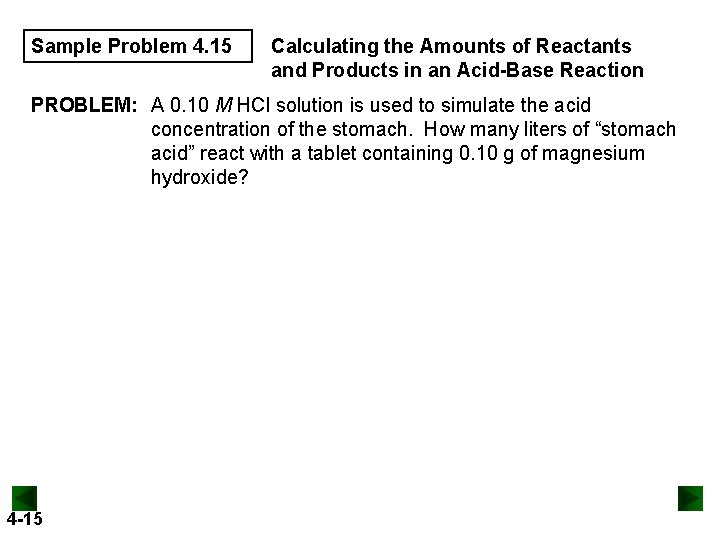
Sample Problem 4. 15 Calculating the Amounts of Reactants and Products in an Acid-Base Reaction PROBLEM: A 0. 10 M HCl solution is used to simulate the acid concentration of the stomach. How many liters of “stomach acid” react with a tablet containing 0. 10 g of magnesium hydroxide? 4 -15
Sample Problem 4. 16 Finding the Concentration of Acid from a Titration PROBLEM: A 50. 00 m. L sample of HCl is titrated with 0. 1524 M Na. OH. The buret reads 0. 55 m. L at the start and 33. 87 m. L at the end point. Find the molarity of the HCl solution. 4 -16

Oxidation-Reduction (Redox) Reactions Oxidation is the loss of electrons. Reduction is the gain of electrons. A redox reaction involves electron transfer. Oxidation and reduction occur together. 4 -17

Figure 4. 16 4 -18 The redox process in compound formation.

Table 4. 3 Rules for Assigning an Oxidation Number (O. N. ) General rules 1. For an atom in its elemental form (Na, O 2, Cl 2, etc. ): O. N. = 0 2. For a monoatomic ion: O. N. = ion charge 3. The sum of O. N. values for the atoms in a compound equals zero. The sum of O. N. values for the atoms in a polyatomic ion equals the ion’s charge. Rules for Specific Atoms or Periodic Table Groups 1. For Group 1 A(1): O. N. = +1 in all compounds 2. For Group 2 A(2): O. N. = +2 in all compounds 3. For hydrogen: O. N. = +1 in combination with nonmetals O. N. = -1 in combination with metals and boron O. N. = -1 in all compounds O. N. = -1 in peroxides O. N. = -2 in all other compounds(except with F) O. N. = -1 in combination with metals, nonmetals (except O), and other halogens lower in the group 4. For fluorine: 5. For oxygen: 6. For Group 7 A(17): 4 -19
Sample Problem 4. 17 Determining the Oxidation Number of Each Element in a Compound (or Ion) PROBLEM: Determine the oxidation number (O. N. ) of each element in these species: (a) zinc chloride (b) sulfur trioxide (c) nitric acid 4 -20

Sample Problem 4. 18 Assign oxidation numbers to the following equation 2 Al(s) + 3 H 2 SO 4(aq) → Al 2 (SO 4)3(aq) + 3 H 2(g) 4 -21
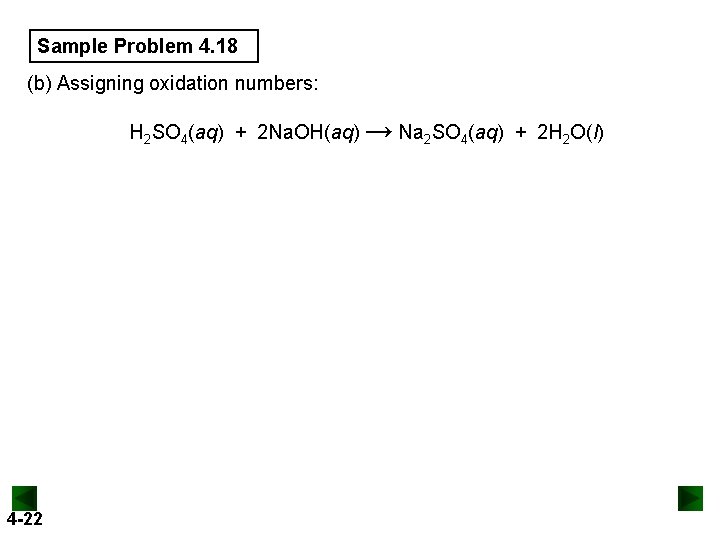
Sample Problem 4. 18 (b) Assigning oxidation numbers: H 2 SO 4(aq) + 2 Na. OH(aq) → Na 2 SO 4(aq) + 2 H 2 O(l) 4 -22
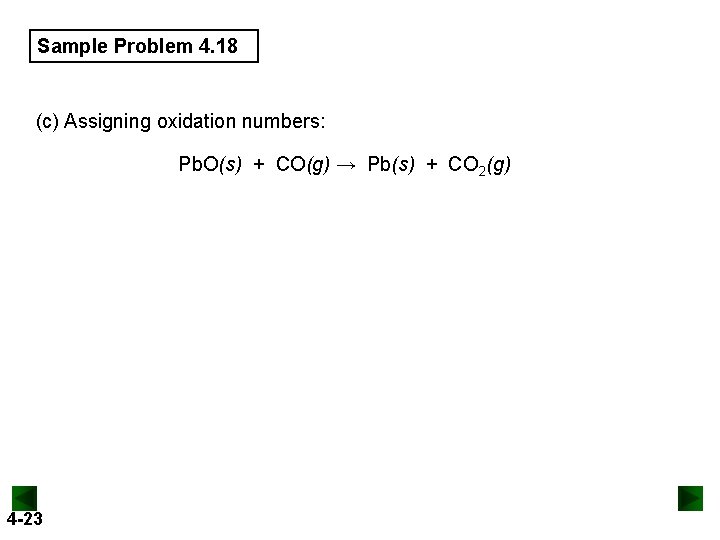
Sample Problem 4. 18 (c) Assigning oxidation numbers: Pb. O(s) + CO(g) → Pb(s) + CO 2(g) 4 -23
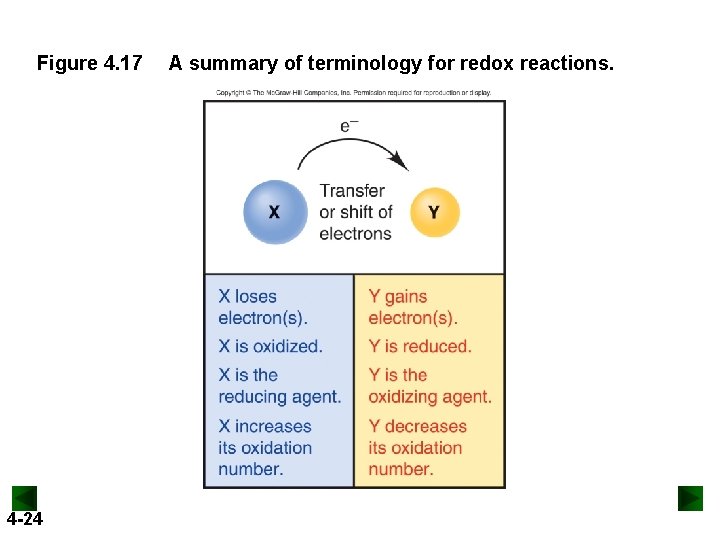
Figure 4. 17 4 -24 A summary of terminology for redox reactions.
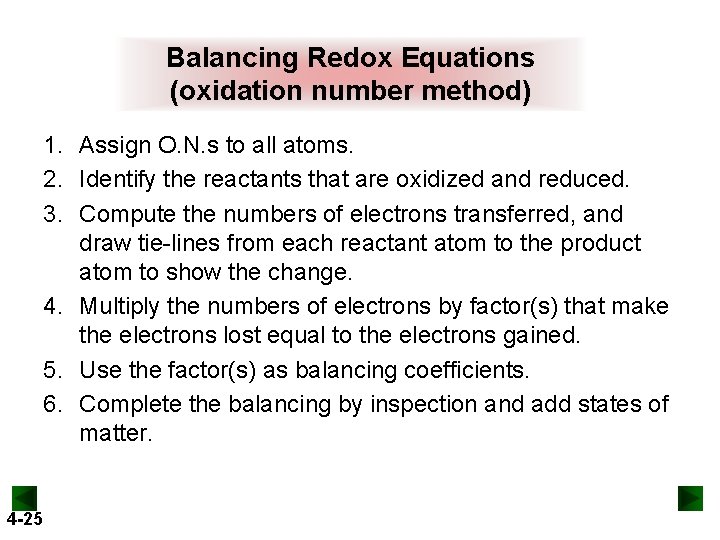
Balancing Redox Equations (oxidation number method) 1. Assign O. N. s to all atoms. 2. Identify the reactants that are oxidized and reduced. 3. Compute the numbers of electrons transferred, and draw tie-lines from each reactant atom to the product atom to show the change. 4. Multiply the numbers of electrons by factor(s) that make the electrons lost equal to the electrons gained. 5. Use the factor(s) as balancing coefficients. 6. Complete the balancing by inspection and add states of matter. 4 -25

Cr 2 O 72 - (aq) 4 -26 + Cl- (aq) Cr 3+ (aq) + Cl 2 (g) acidic solution

Cu (s) 4 -27 + NO 3 - (aq) Cu 2+ (aq) + NO 2 (g) acidic solution
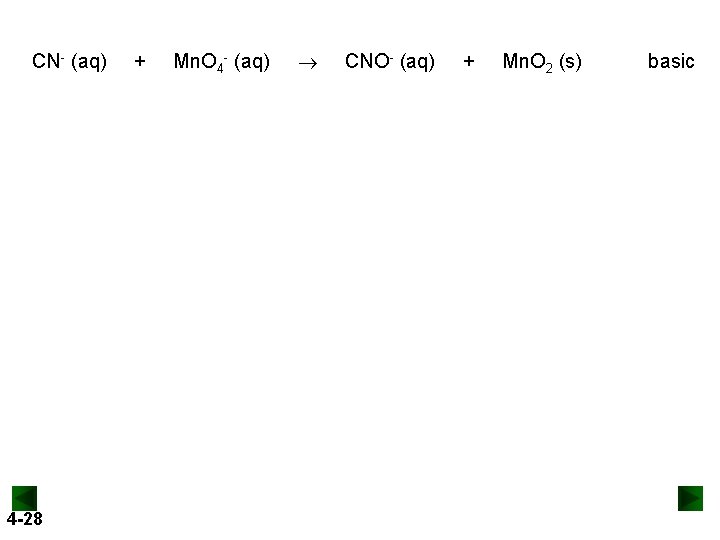
CN- (aq) 4 -28 + Mn. O 4 - (aq) CNO- (aq) + Mn. O 2 (s) basic

NO 2 - (aq) 4 -29 + Al (s) NH 3 (aq) + Al(OH)4 - basic
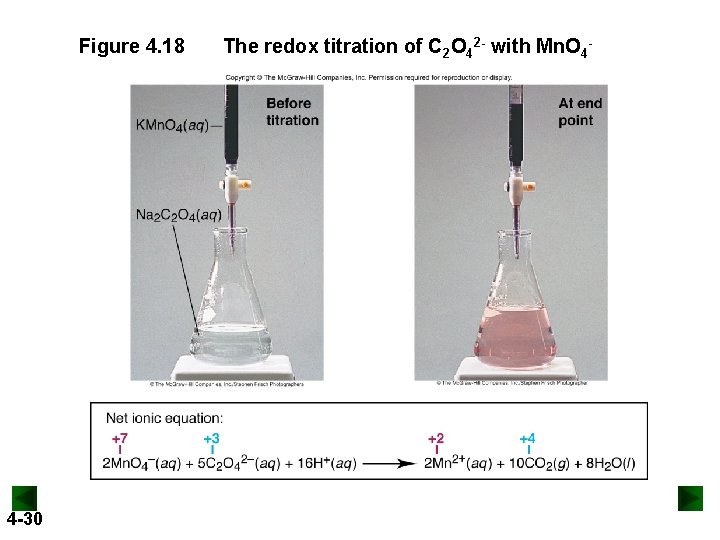
Figure 4. 18 4 -30 The redox titration of C 2 O 42 - with Mn. O 4 -
Sample Problem 4. 19 Finding the Amount of Reducing Agent by Titration PROBLEM: To measure the Ca 2+ concentration in human blood, 1. 00 m. L of blood was treated with Na 2 C 2 O 4 solution to precipitate the Ca 2+ as Ca. C 2 O 4. The precipitate was filtered and dissolved in dilute H 2 SO 4 to release C 2 O 42 -, which was titrated with KMn. O 4 solution. The solution required 2. 05 m. L of 4. 88 x 10 -4 M KMn. O 4 to reach the end point. The balanced equation is 2 KMn. O 4 (aq) + 5 Ca. C 2 O 4 (s) + 8 H 2 SO 4 (aq) → 2 Mn. SO 4 (aq) + K 2 SO 4 (aq) + 5 Ca. SO 4 (s) + 10 CO 2 (g) + 8 H 2 O (l) Calculate the amount (mol) of Ca 2+ in 1. 00 m. L of blood. 4 -31

2 KMn. O 4 (aq) + 5 Ca. C 2 O 4 (s) + 8 H 2 SO 4 (aq) → 2 Mn. SO 4 (aq) + K 2 SO 4 (aq) + 5 Ca. SO 4 (s) + 10 CO 2 (g) + 8 H 2 O (l) 4 -32
Elements in Redox Reactions Types of Reactions • Combination Reactions – Two or more reactants combine to form a new compound: – X+Y→Z • Decomposition Reactions – A single compound decomposes to form two or more products: – Z→X+Y • Displacement Reactions – double diplacement: AB + CD → AD + CB – single displacement: X + YZ → XZ + Y • Combustion – the process of combining with O 2 4 -33
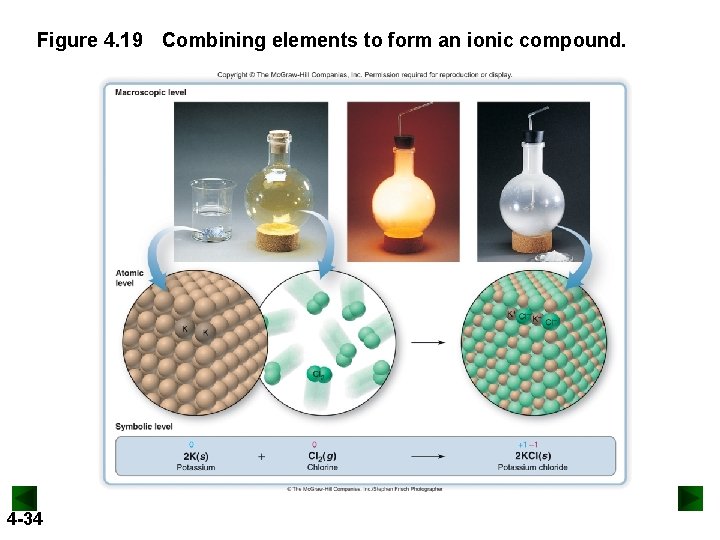
Figure 4. 19 Combining elements to form an ionic compound. 4 -34
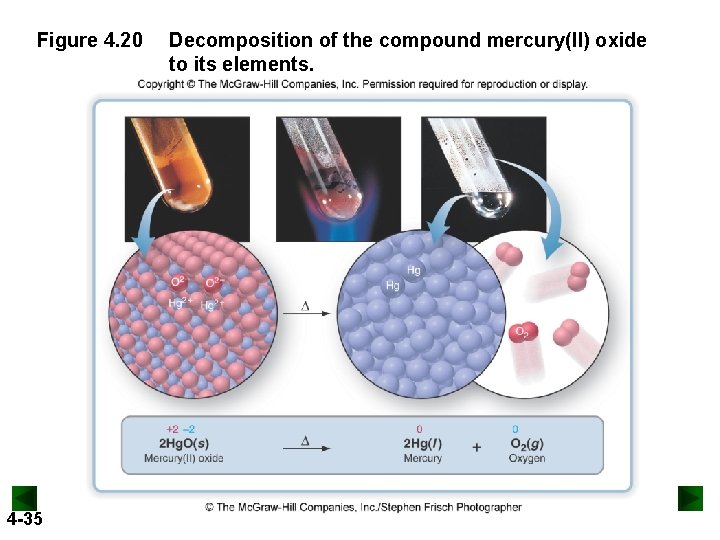
Figure 4. 20 4 -35 Decomposition of the compound mercury(II) oxide to its elements.
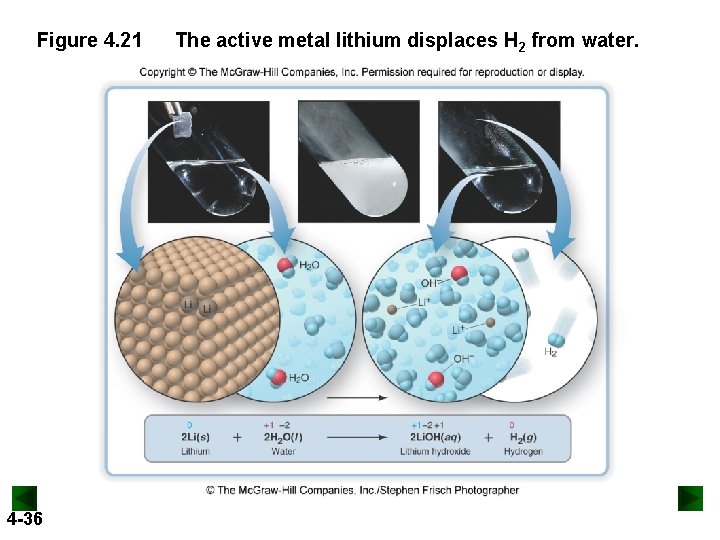
Figure 4. 21 4 -36 The active metal lithium displaces H 2 from water.
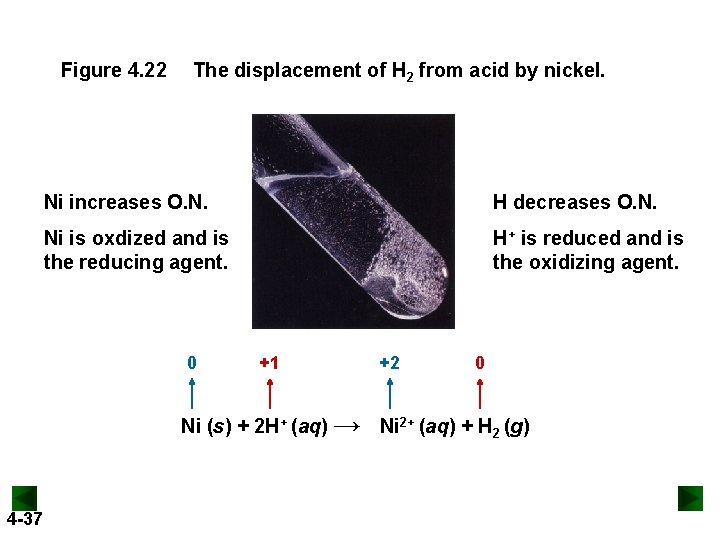
Figure 4. 22 The displacement of H 2 from acid by nickel. Ni increases O. N. H decreases O. N. Ni is oxdized and is the reducing agent. H+ is reduced and is the oxidizing agent. 0 +1 +2 0 Ni (s) + 2 H+ (aq) → Ni 2+ (aq) + H 2 (g) 4 -37
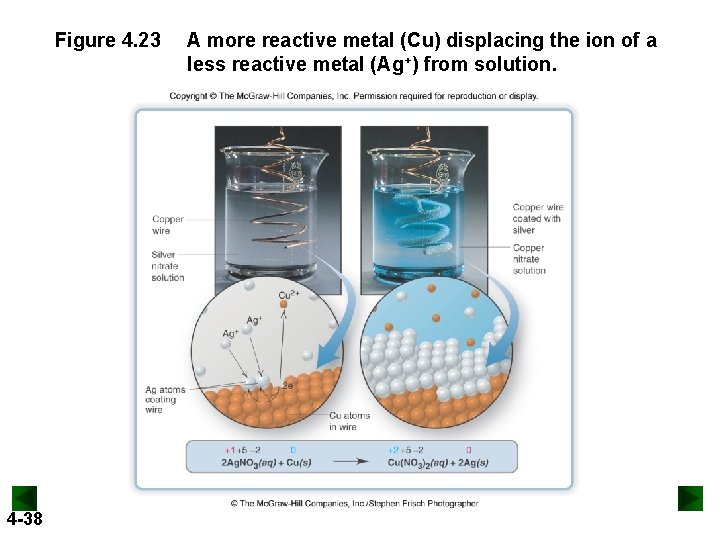
Figure 4. 23 4 -38 A more reactive metal (Cu) displacing the ion of a less reactive metal (Ag+) from solution.
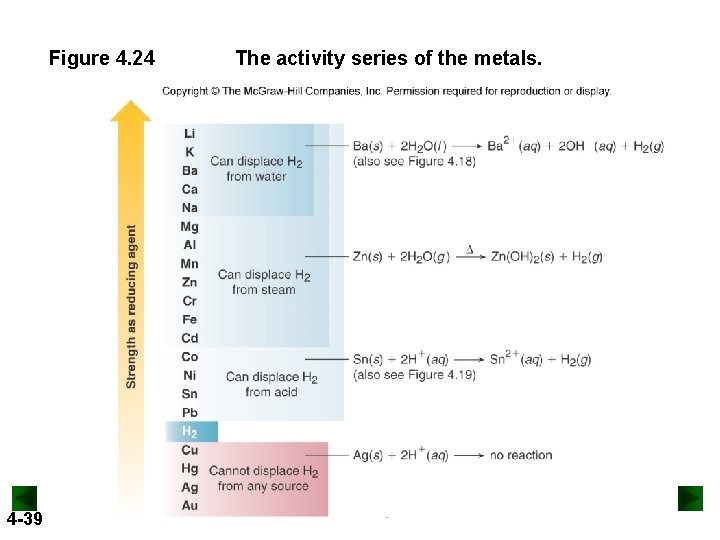
Figure 4. 24 4 -39 The activity series of the metals.
Sample Problem 4. 20 Identifying the Type of Redox Reaction PROBLEM: Classify each of the following redox reactions as a combination, decomposition, or displacement reaction. Write a balanced molecular equation for each, as well as total and net ionic equations for part (c), and identify the oxidizing and reducing agents: (a) magnesium (s) + nitrogen (g) → magnesium nitride (s) (b) hydrogen peroxide (l) → water (l) + oxygen gas (c) aluminum (s) + lead(II) nitrate (aq) → aluminum nitrate (aq) + lead (s) 4 -40

(a) magnesium (s) + nitrogen (g) → magnesium nitride (s) 4 -41

(b) hydrogen peroxide (l) → water (l) + oxygen gas 4 -42

(c) aluminum (s) + lead(II) nitrate (aq) → aluminum nitrate (aq) + lead (s) 4 -43
- Slides: 43