AcidBase Equilibria and Solubility Equilibria Chapter 16 1

Acid-Base Equilibria and Solubility Equilibria Chapter 16 1 Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display.
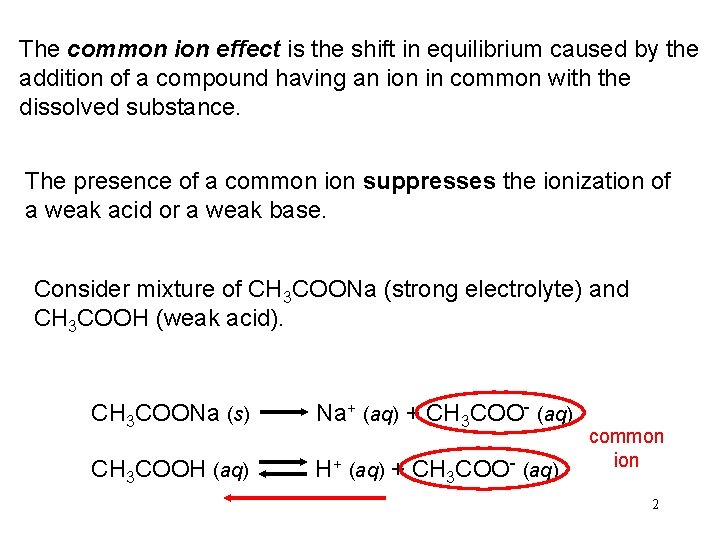
The common ion effect is the shift in equilibrium caused by the addition of a compound having an ion in common with the dissolved substance. The presence of a common ion suppresses the ionization of a weak acid or a weak base. Consider mixture of CH 3 COONa (strong electrolyte) and CH 3 COOH (weak acid). CH 3 COONa (s) Na+ (aq) + CH 3 COO- (aq) CH 3 COOH (aq) H+ (aq) + CH 3 COO- (aq) common ion 2
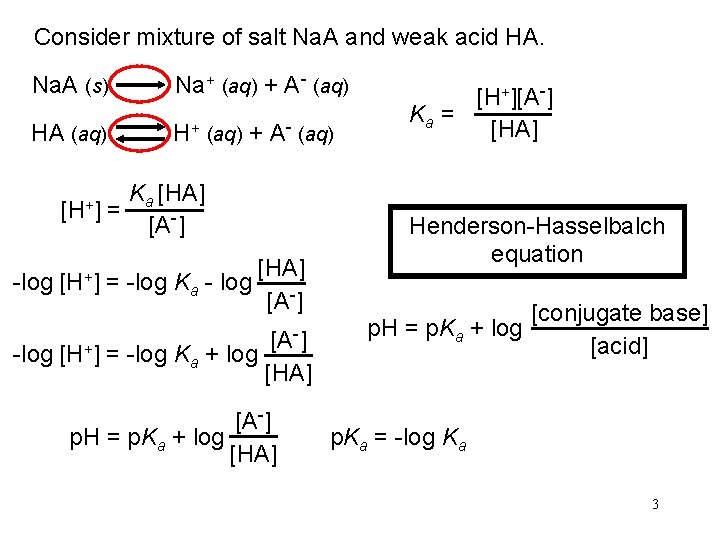
Consider mixture of salt Na. A and weak acid HA. Na. A (s) Na+ (aq) + A- (aq) HA (aq) H+ (aq) + A- (aq) [H+] = Ka [HA] [A-] -log [H+] = -log Ka - log [HA] [A-] -] [A -log [H+] = -log Ka + log [HA] [A-] p. H = p. Ka + log [HA] [H+][A-] Ka = [HA] Henderson-Hasselbalch equation p. H = p. Ka + log [conjugate base] [acid] p. Ka = -log Ka 3
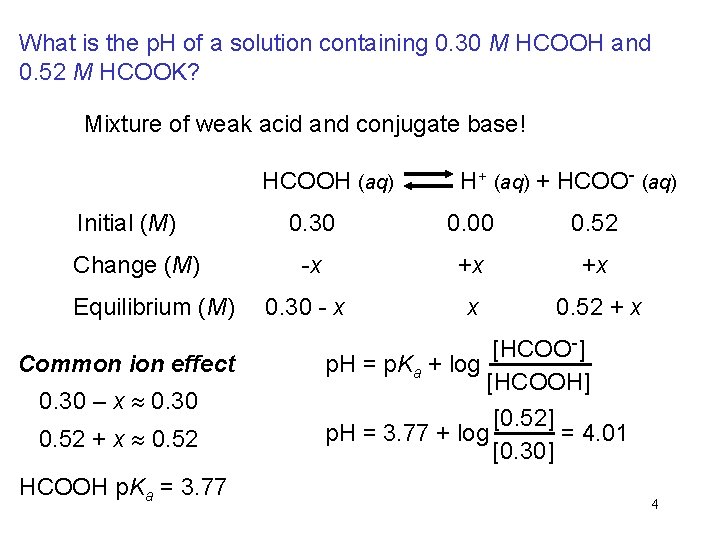
What is the p. H of a solution containing 0. 30 M HCOOH and 0. 52 M HCOOK? Mixture of weak acid and conjugate base! HCOOH (aq) H+ (aq) + HCOO- (aq) Initial (M) Change (M) Equilibrium (M) Common ion effect 0. 30 – x 0. 30 0. 52 + x 0. 52 HCOOH p. Ka = 3. 77 0. 30 0. 00 0. 52 -x +x +x 0. 30 - x x 0. 52 + x [HCOO-] p. H = p. Ka + log [HCOOH] [0. 52] = 4. 01 p. H = 3. 77 + log [0. 30] 4

A buffer solution is a solution of: 1. A weak acid or a weak base and 2. The salt of the weak acid or weak base Both must be present! A buffer solution has the ability to resist changes in p. H upon the addition of small amounts of either acid or base. Consider an equal molar mixture of CH 3 COOH and CH 3 COONa Add strong acid H+ (aq) + CH 3 COO- (aq) CH 3 COOH (aq) Add strong base OH- (aq) + CH 3 COOH (aq) CH 3 COO- (aq) + H 2 O (l) 5
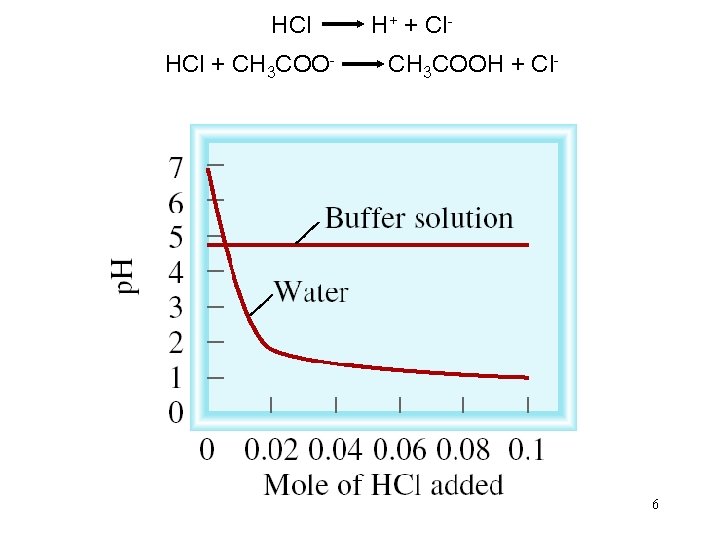
HCl H+ + Cl. HCl + CH 3 COO- CH 3 COOH + Cl- 6

Which of the following are buffer systems? (a) KF/HF (b) KBr/HBr, (c) Na 2 CO 3/Na. HCO 3 (a) KF is a weak acid and F- is its conjugate base buffer solution (b) HBr is a strong acid not a buffer solution (c) CO 32 - is a weak base and HCO 3 - is its conjugate acid buffer solution 7
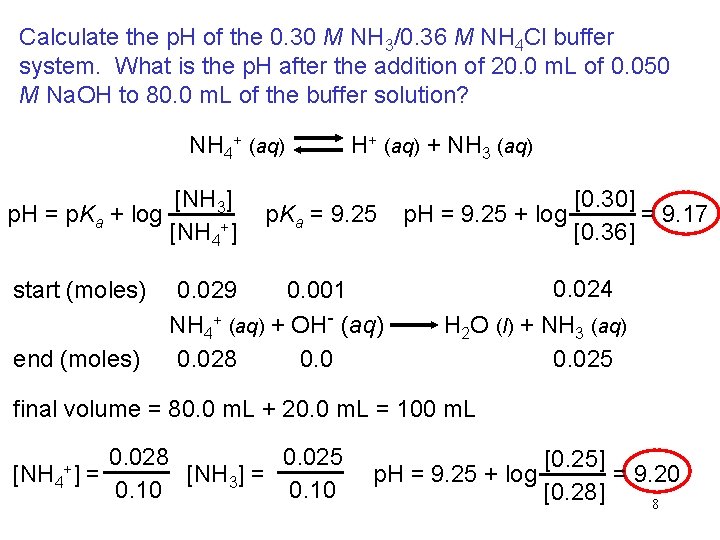
Calculate the p. H of the 0. 30 M NH 3/0. 36 M NH 4 Cl buffer system. What is the p. H after the addition of 20. 0 m. L of 0. 050 M Na. OH to 80. 0 m. L of the buffer solution? NH 4+ (aq) H+ (aq) + NH 3 (aq) [NH 3] p. H = p. Ka + log [NH 4+] start (moles) end (moles) p. Ka = 9. 25 [0. 30] p. H = 9. 25 + log = 9. 17 [0. 36] 0. 024 0. 029 0. 001 NH 4+ (aq) + OH- (aq) H 2 O (l) + NH 3 (aq) 0. 028 0. 025 final volume = 80. 0 m. L + 20. 0 m. L = 100 m. L [NH 4 +] = 0. 028 0. 025 [NH 3] = 0. 10 [0. 25] = 9. 20 p. H = 9. 25 + log [0. 28] 8
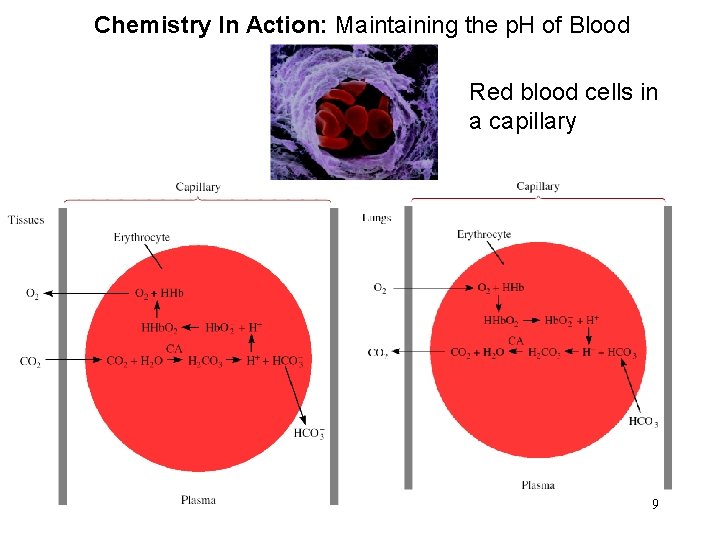
Chemistry In Action: Maintaining the p. H of Blood Red blood cells in a capillary 9
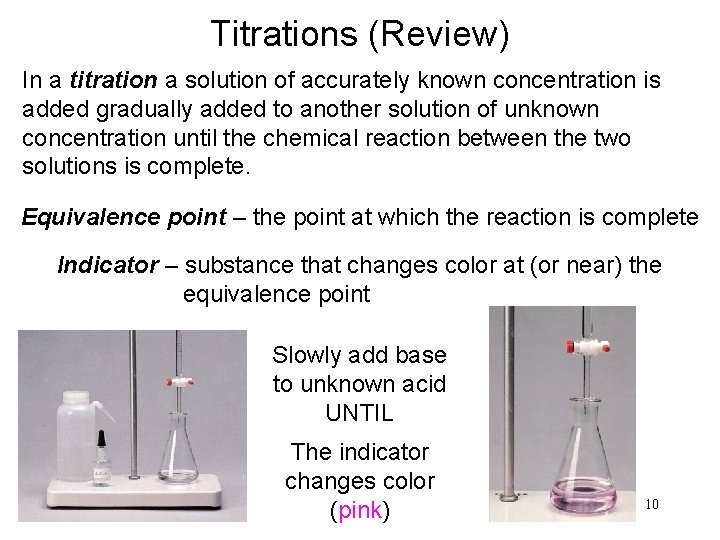
Titrations (Review) In a titration a solution of accurately known concentration is added gradually added to another solution of unknown concentration until the chemical reaction between the two solutions is complete. Equivalence point – the point at which the reaction is complete Indicator – substance that changes color at (or near) the equivalence point Slowly add base to unknown acid UNTIL The indicator changes color (pink) 10

Alternative Method of Equivalence Point Detection monitor p. H 11
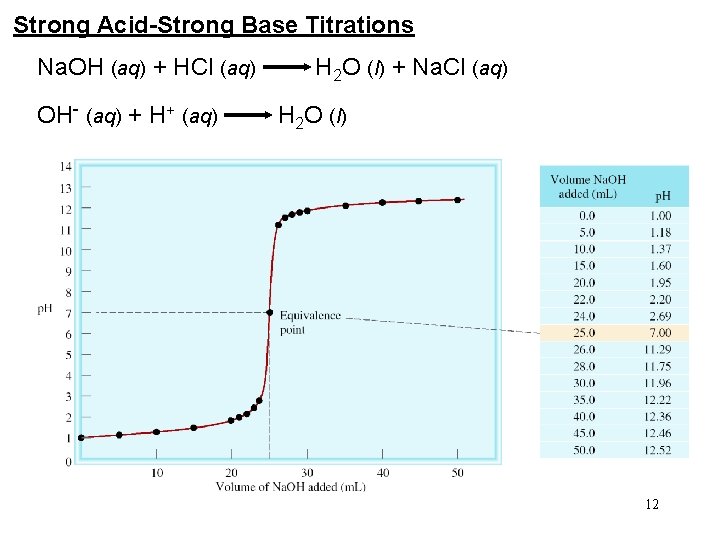
Strong Acid-Strong Base Titrations Na. OH (aq) + HCl (aq) H 2 O (l) + Na. Cl (aq) OH- (aq) + H+ (aq) H 2 O (l) 12
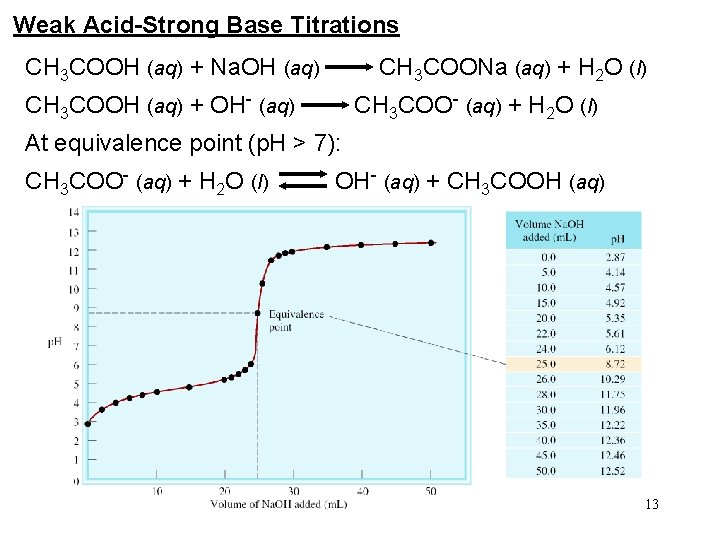
Weak Acid-Strong Base Titrations CH 3 COOH (aq) + Na. OH (aq) CH 3 COONa (aq) + H 2 O (l) CH 3 COOH (aq) + OH- (aq) CH 3 COO- (aq) + H 2 O (l) At equivalence point (p. H > 7): CH 3 COO- (aq) + H 2 O (l) OH- (aq) + CH 3 COOH (aq) 13
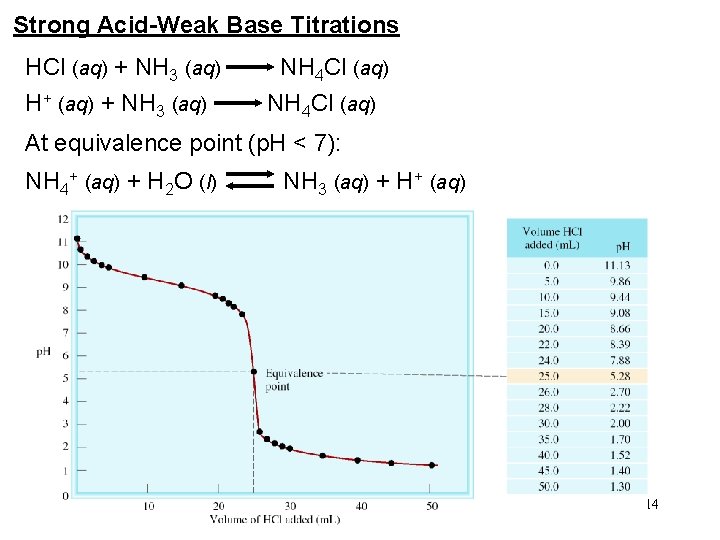
Strong Acid-Weak Base Titrations HCl (aq) + NH 3 (aq) NH 4 Cl (aq) H+ (aq) + NH 3 (aq) NH 4 Cl (aq) At equivalence point (p. H < 7): NH 4+ (aq) + H 2 O (l) NH 3 (aq) + H+ (aq) 14
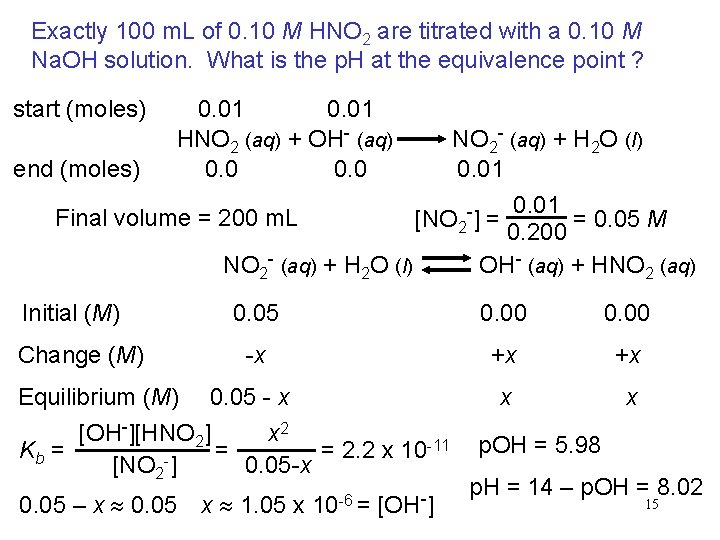
Exactly 100 m. L of 0. 10 M HNO 2 are titrated with a 0. 10 M Na. OH solution. What is the p. H at the equivalence point ? start (moles) 0. 01 HNO 2 (aq) + OH- (aq) NO 2 - (aq) + H 2 O (l) end (moles) 0. 01 Final volume = 200 m. L [NO 2 -] = = 0. 05 M 0. 200 NO 2 - (aq) + H 2 O (l) OH- (aq) + HNO 2 (aq) Initial (M) Change (M) Equilibrium (M) 0. 05 0. 00 -x +x +x 0. 05 - x x x [OH-][HNO 2] x 2 -11 = 2. 2 x 10 Kb = = [NO 2 -] 0. 05 -x 0. 05 – x 0. 05 x 1. 05 x 10 -6 = [OH-] p. OH = 5. 98 p. H = 14 – p. OH = 8. 02 15
![Acid-Base Indicators HIn (aq) H+ (aq) + In- (aq) [HIn] 10 Color of acid Acid-Base Indicators HIn (aq) H+ (aq) + In- (aq) [HIn] 10 Color of acid](http://slidetodoc.com/presentation_image_h/1d890f9871f75dae50591b1fbed0110d/image-16.jpg)
Acid-Base Indicators HIn (aq) H+ (aq) + In- (aq) [HIn] 10 Color of acid (HIn) predominates [In ] [HIn] -) predominates Color of conjugate base (In 10 [In-] 16
Solutions of Red Cabbage Extract p. H 17
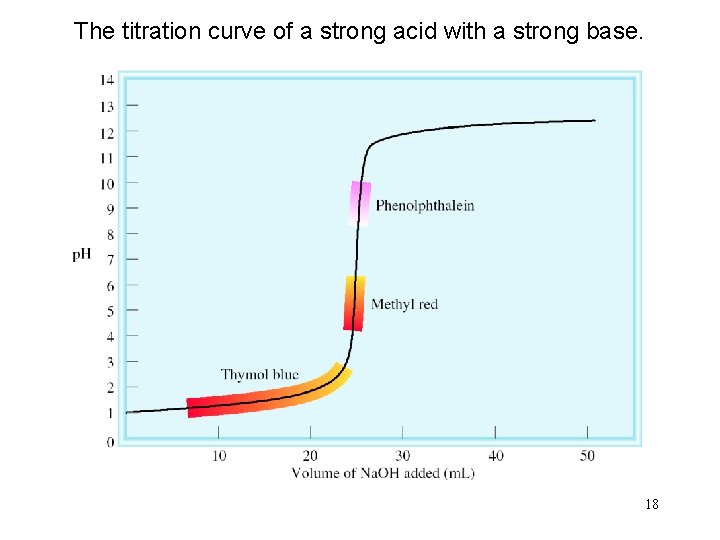
The titration curve of a strong acid with a strong base. 18

Which indicator(s) would you use for a titration of HNO 2 with KOH ? Weak acid titrated with strong base. At equivalence point, will have conjugate base of weak acid. At equivalence point, p. H > 7 Use cresol red or phenolphthalein 19
![Solubility Equilibria Ag. Cl (s) Ag+ (aq) + Cl- (aq) Ksp = [Ag+][Cl-] Ksp Solubility Equilibria Ag. Cl (s) Ag+ (aq) + Cl- (aq) Ksp = [Ag+][Cl-] Ksp](http://slidetodoc.com/presentation_image_h/1d890f9871f75dae50591b1fbed0110d/image-20.jpg)
Solubility Equilibria Ag. Cl (s) Ag+ (aq) + Cl- (aq) Ksp = [Ag+][Cl-] Ksp is the solubility product constant Mg. F 2 (s) Mg 2+ (aq) + 2 F- (aq) Ksp = [Mg 2+][F-]2 Ag 2 CO 3 (s) 2 Ag+ (aq) + CO 32 - (aq) Ksp = [Ag+]2[CO 32 -] Ca 3(PO 4)2 (s) 3 Ca 2+ (aq) + 2 PO 43 - (aq) Ksp = [Ca 2+]3[PO 43 -]2 Dissolution of an ionic solid in aqueous solution: Q < Ksp Unsaturated solution Q = Ksp Saturated solution Q > Ksp Supersaturated solution No precipitate Precipitate will form 20
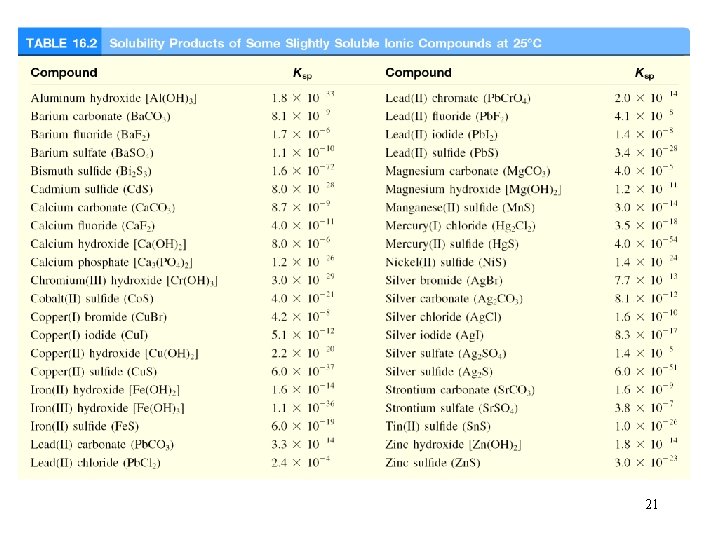
21
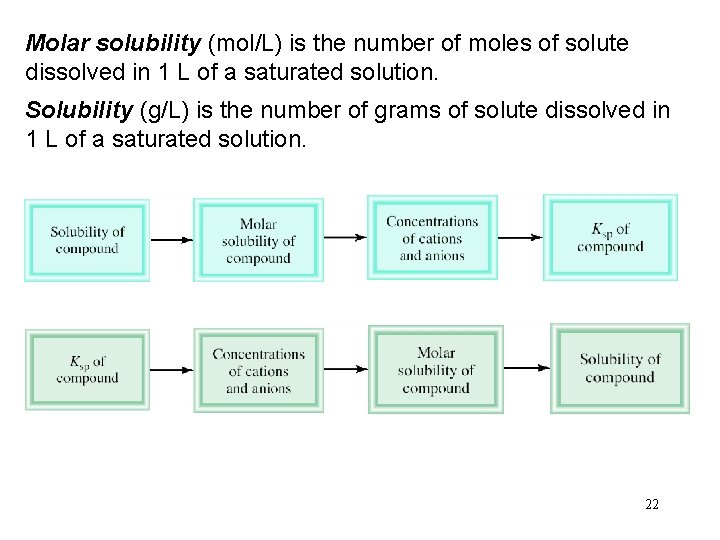
Molar solubility (mol/L) is the number of moles of solute dissolved in 1 L of a saturated solution. Solubility (g/L) is the number of grams of solute dissolved in 1 L of a saturated solution. 22

What is the solubility of silver chloride in g/L ? Initial (M) Ag. Cl (s) Ag+ (aq) + Cl- (aq) 0. 00 Change (M) Equilibrium (M) [Ag+] = 1. 3 x 10 -5 M +s +s s s [Cl-] = 1. 3 x 10 -5 M Ksp = 1. 6 x 10 -10 Ksp = [Ag+][Cl-] Ksp = s 2 s = K sp s = 1. 3 x 10 -5 mol Ag. Cl 143. 35 g Ag. Cl Solubility of Ag. Cl = x = 1. 9 x 10 -3 g/L 1 L soln 1 mol Ag. Cl 23
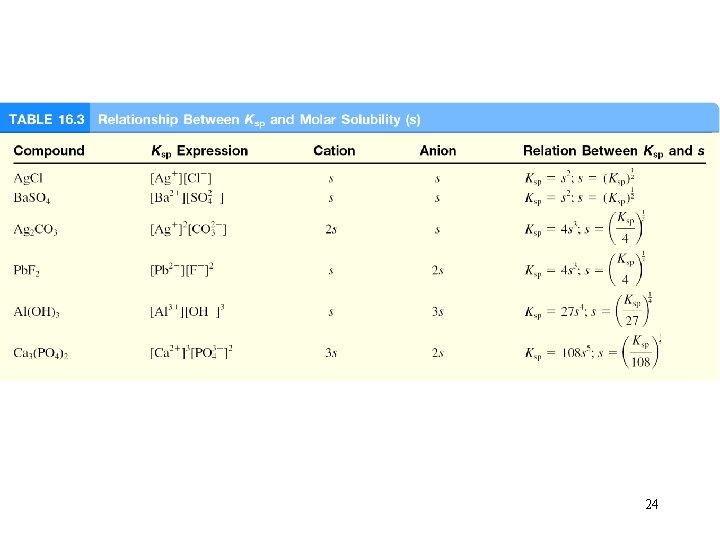
24
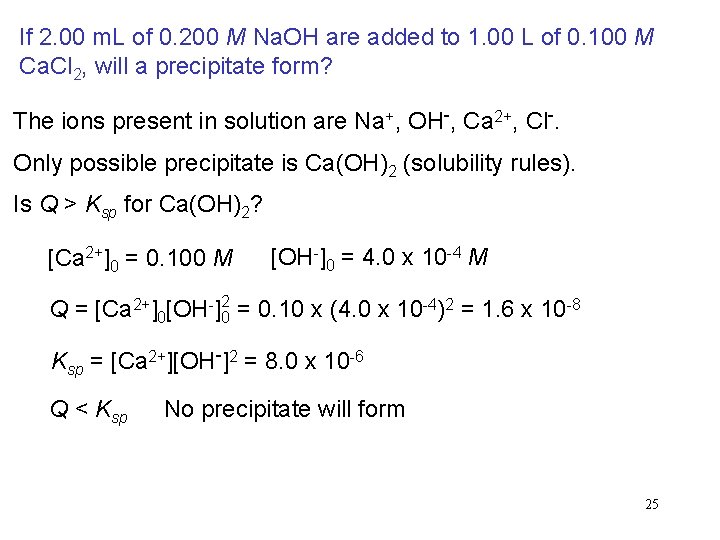
If 2. 00 m. L of 0. 200 M Na. OH are added to 1. 00 L of 0. 100 M Ca. Cl 2, will a precipitate form? The ions present in solution are Na+, OH-, Ca 2+, Cl-. Only possible precipitate is Ca(OH)2 (solubility rules). Is Q > Ksp for Ca(OH)2? [Ca 2+]0 = 0. 100 M [OH-]0 = 4. 0 x 10 -4 M Q = [Ca 2+]0[OH-]02 = 0. 10 x (4. 0 x 10 -4)2 = 1. 6 x 10 -8 Ksp = [Ca 2+][OH-]2 = 8. 0 x 10 -6 Q < Ksp No precipitate will form 25
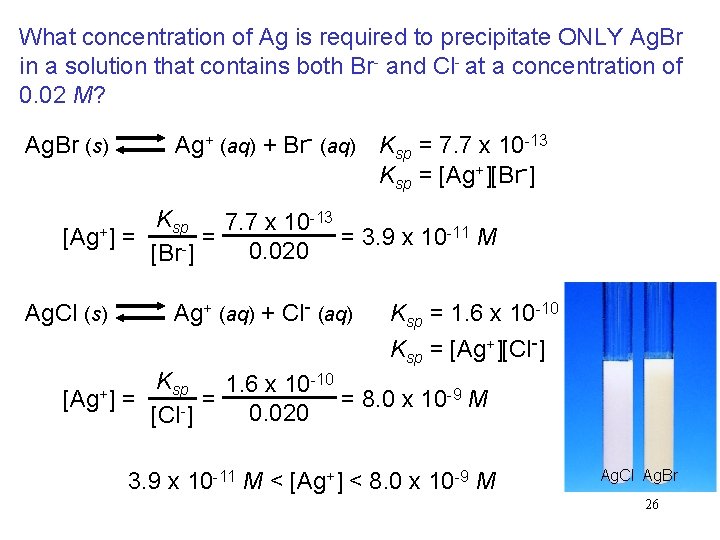
What concentration of Ag is required to precipitate ONLY Ag. Br in a solution that contains both Br- and Cl- at a concentration of 0. 02 M? Ag. Br (s) Ag+ (aq) + Br- (aq) Ksp = 7. 7 x 10 -13 Ksp = [Ag+][Br-] -13 K 7. 7 x 10 sp -11 M = = 3. 9 x 10 [Ag+] = 0. 020 [Br-] Ag. Cl (s) Ag+ (aq) + Cl- (aq) [Ag+] = Ksp = 1. 6 x 10 -10 Ksp = [Ag+][Cl-] Ksp 1. 6 x 10 -10 -9 M = = 8. 0 x 10 0. 020 [Cl-] 3. 9 x 10 -11 M < [Ag+] < 8. 0 x 10 -9 M Ag. Cl Ag. Br 26
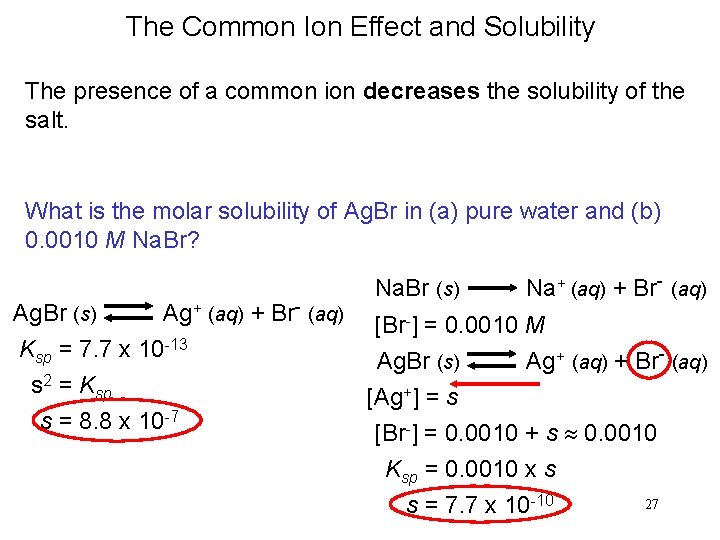
The Common Ion Effect and Solubility The presence of a common ion decreases the solubility of the salt. What is the molar solubility of Ag. Br in (a) pure water and (b) 0. 0010 M Na. Br? Na. Br (s) Na+ (aq) + Br- (aq) Ag. Br (s) Ag+ (aq) + Br- (aq) [Br-] = 0. 0010 M Ksp = 7. 7 x 10 -13 Ag. Br (s) Ag+ (aq) + Br- (aq) s 2 = Ksp [Ag+] = s s = 8. 8 x 10 -7 [Br-] = 0. 0010 + s 0. 0010 Ksp = 0. 0010 x s s = 7. 7 x 10 -10 27
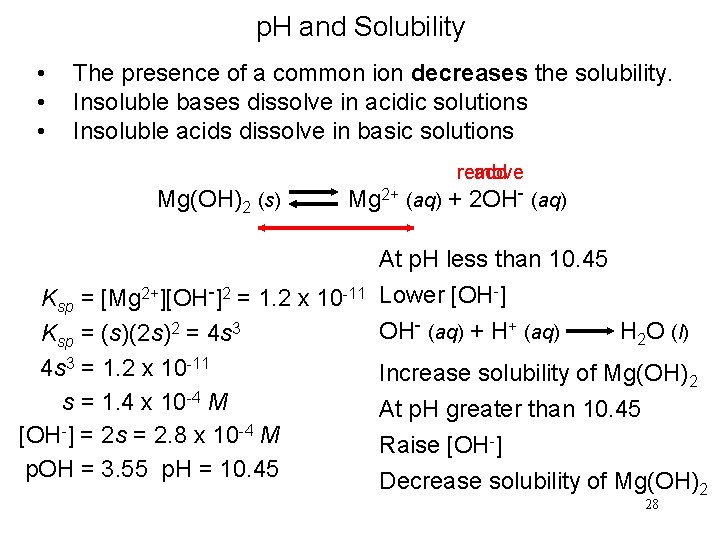
p. H and Solubility • • • The presence of a common ion decreases the solubility. Insoluble bases dissolve in acidic solutions Insoluble acids dissolve in basic solutions remove add Mg(OH)2 (s) Mg 2+ (aq) + 2 OH- (aq) Ksp = [Mg 2+][OH-]2 = 1. 2 x 10 -11 Ksp = (s)(2 s)2 = 4 s 3 = 1. 2 x 10 -11 s = 1. 4 x 10 -4 M [OH-] = 2 s = 2. 8 x 10 -4 M p. OH = 3. 55 p. H = 10. 45 At p. H less than 10. 45 Lower [OH-] OH- (aq) + H+ (aq) H 2 O (l) Increase solubility of Mg(OH)2 At p. H greater than 10. 45 Raise [OH-] Decrease solubility of Mg(OH)2 28
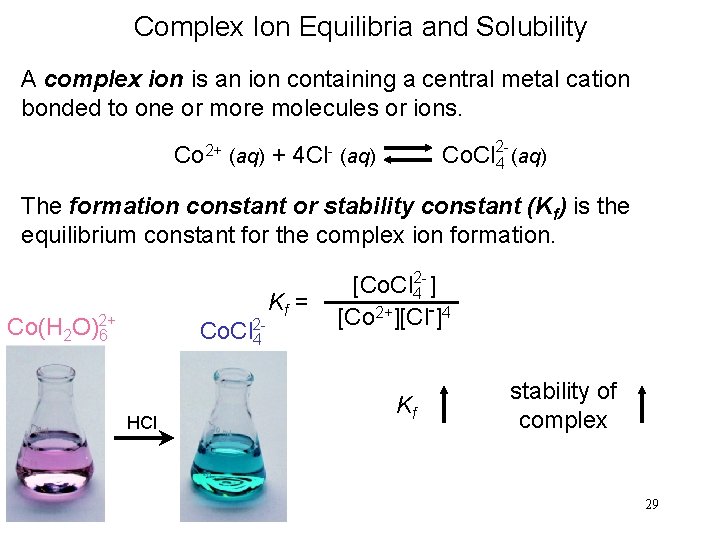
Complex Ion Equilibria and Solubility A complex ion is an ion containing a central metal cation bonded to one or more molecules or ions. Co 2+ (aq) + 4 Cl- (aq) Co. Cl 24 (aq) The formation constant or stability constant (Kf) is the equilibrium constant for the complex ion formation. Co(H 2 O)2+ 6 Co. Cl 24 HCl Kf = [Co. Cl 42 - ] [Co 2+][Cl-]4 Kf stability of complex 29

Effect of Complexation on Solubility Ag. NO Add NH 3 + Na. Cl 3 Ag(NH Ag. Cl 3)2+ 30
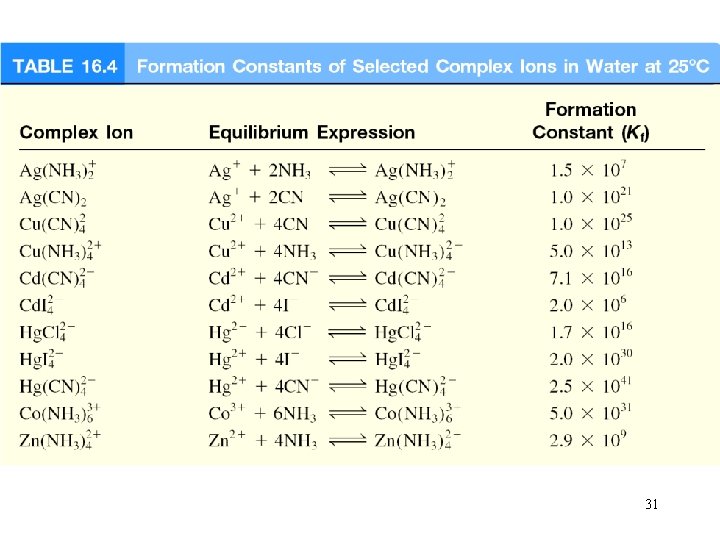
31
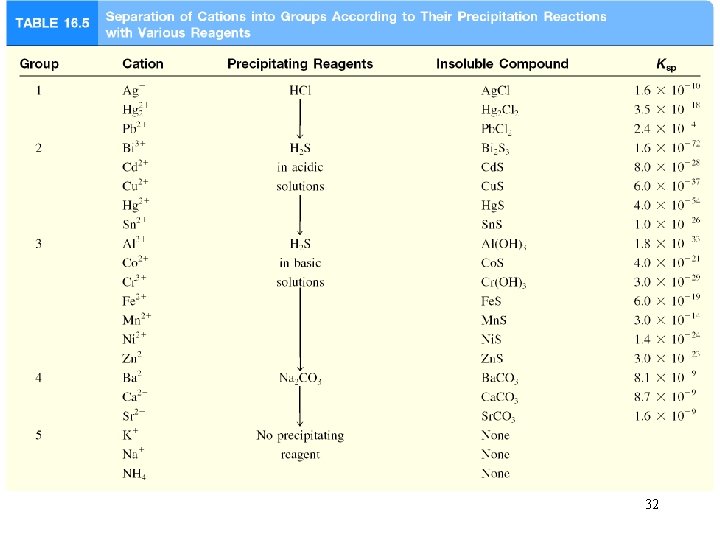
32
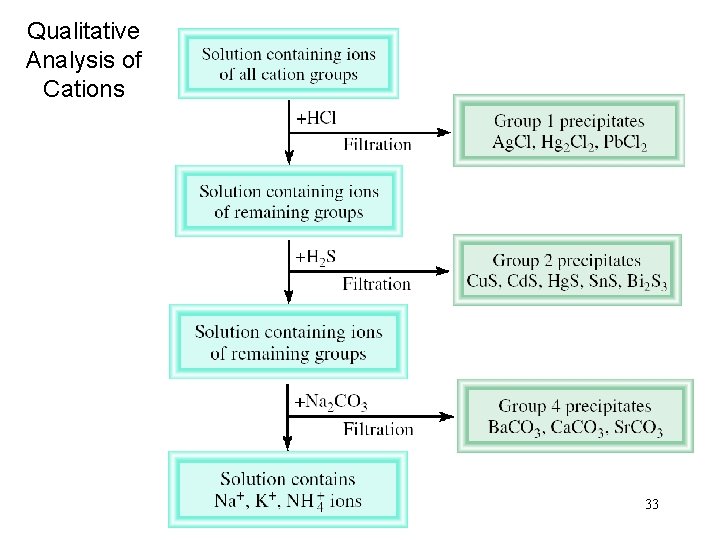
Qualitative Analysis of Cations 33
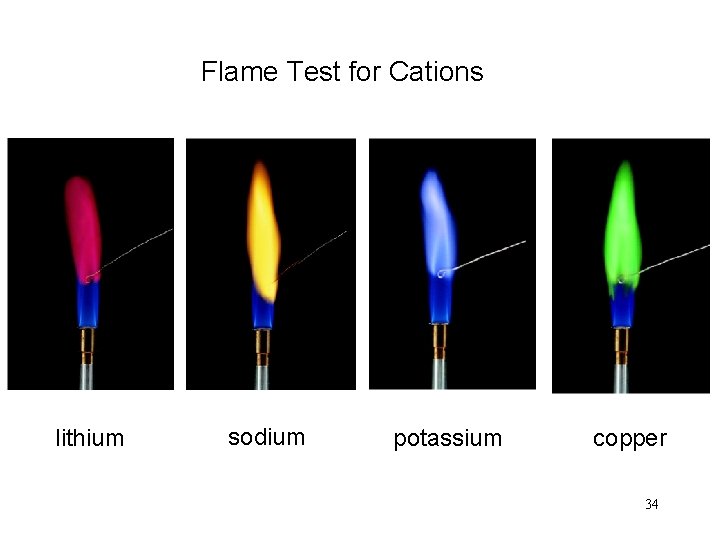
Flame Test for Cations lithium sodium potassium copper 34
Chemistry In Action: How an Eggshell is Formed Ca 2+ (aq) + CO 32 - (aq) Ca. CO 3 (s) carbonic CO 2 (g) + H 2 O (l) H 2 CO 3 (aq) anhydrase H 2 CO 3 (aq) H+ (aq) + HCO 3 - (aq) H+ (aq) + CO 32 - (aq) electron micrograph 35
- Slides: 35