AcidBase Balance Respiratory Acidosis Characterized by an increase

Acid-Base Balance
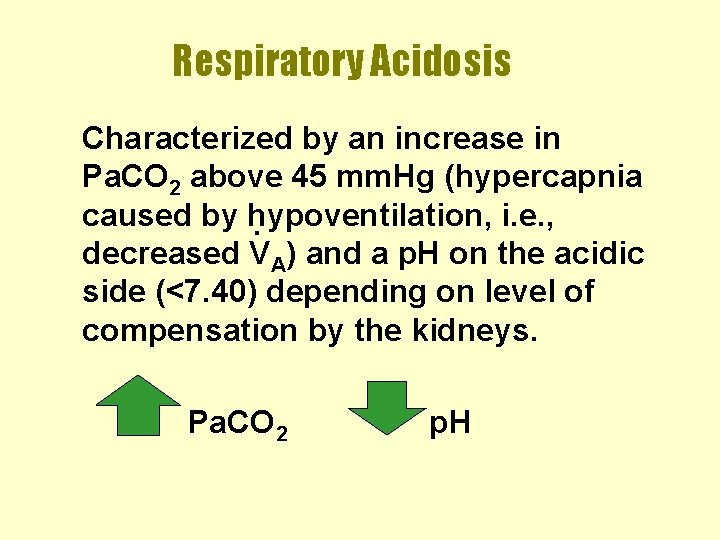
Respiratory Acidosis Characterized by an increase in Pa. CO 2 above 45 mm. Hg (hypercapnia caused by hypoventilation, i. e. , . decreased VA) and a p. H on the acidic side (<7. 40) depending on level of compensation by the kidneys. Pa. CO 2 p. H
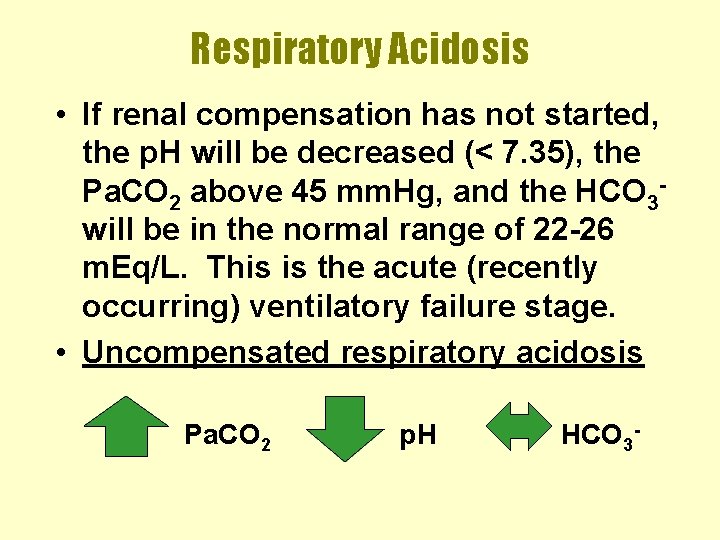
Respiratory Acidosis • If renal compensation has not started, the p. H will be decreased (< 7. 35), the Pa. CO 2 above 45 mm. Hg, and the HCO 3 will be in the normal range of 22 -26 m. Eq/L. This is the acute (recently occurring) ventilatory failure stage. • Uncompensated respiratory acidosis Pa. CO 2 p. H HCO 3 -
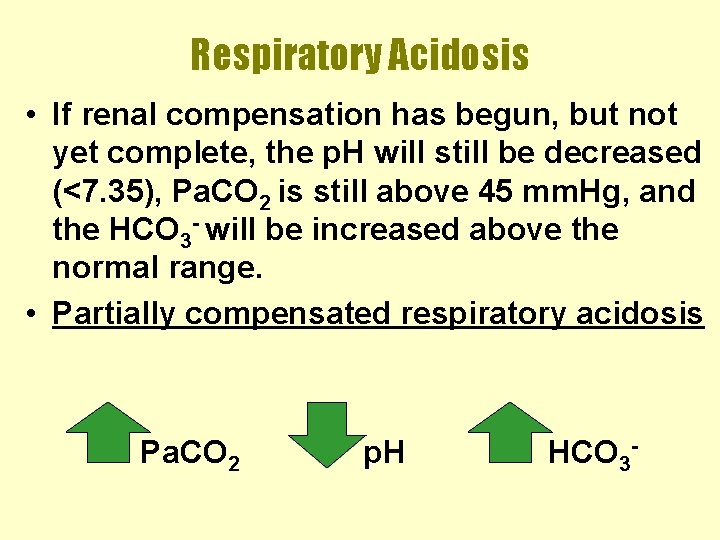
Respiratory Acidosis • If renal compensation has begun, but not yet complete, the p. H will still be decreased (<7. 35), Pa. CO 2 is still above 45 mm. Hg, and the HCO 3 - will be increased above the normal range. • Partially compensated respiratory acidosis Pa. CO 2 p. H HCO 3 -
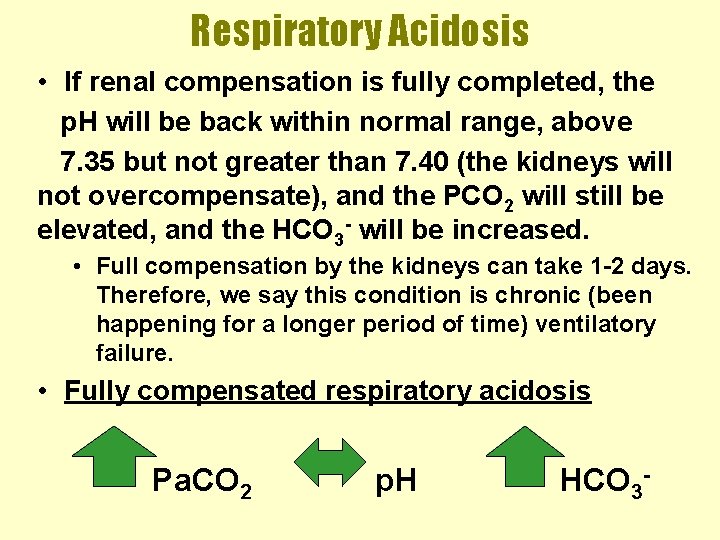
Respiratory Acidosis • If renal compensation is fully completed, the p. H will be back within normal range, above 7. 35 but not greater than 7. 40 (the kidneys will not overcompensate), and the PCO 2 will still be elevated, and the HCO 3 - will be increased. • Full compensation by the kidneys can take 1 -2 days. Therefore, we say this condition is chronic (been happening for a longer period of time) ventilatory failure. • Fully compensated respiratory acidosis Pa. CO 2 p. H HCO 3 -

Respiratory Acidosis • In addition to waiting for renal compensation, it is also possible to correct the respiratory acidosis, i. e. , provide medical intervention to eliminate the ventilatory failure and improve alveolar ventilation. This may involve intubation, mechanical ventilation, and/or breathing treatments by therapist.

Common Causes of Respiratory Acidosis Lung tissue is healthy, but something is interfering with ventilation • CNS depression • Anesthesia, sedative drugs, narcotics • Neuromuscular diseases • Myasthenia gravis, Guillain-Barré syndrome • Trauma to spinal cord, brain, or chest wall • Severe restrictive disorders • Pickwickian disorder, obesity, skeletal abnormalities

Common Causes of Respiratory Acidosis Lung tissue and/or airways are abnormal • COPD – emphysema and chronic bronchitis • Cystic fibrosis • Advanced-stage of pneumonia, asthma, airway obstruction • Massive pulmonary edema • V/Q abnormalities

Clinical Manifestations of Respiratory Acidosis Often depends on underlying problem • Neuromuscular or airway obstruction: SOB, tachypnea • CNS impairment: reduced respiratory rate • Acute hypercapnia: headache, lethargy, confusion, coma, signs of hypoxemia, systemic vasodilation, arrhythmia
Treatment of Respiratory Acidosis Treat the underlying problem
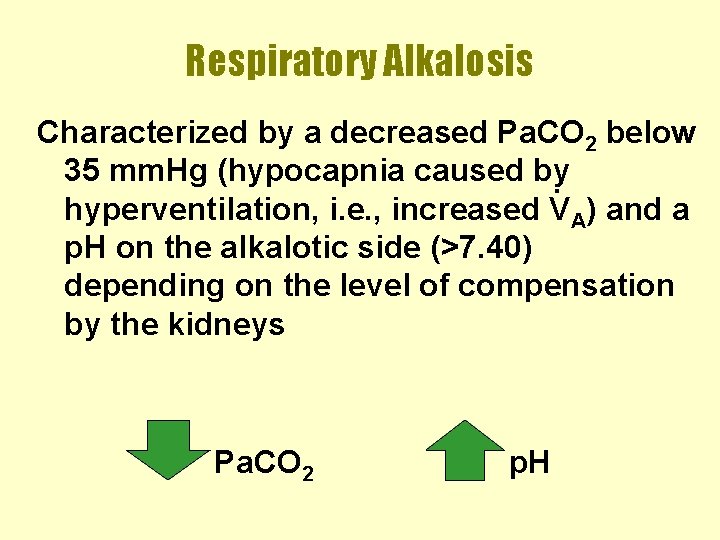
Respiratory Alkalosis Characterized by a decreased Pa. CO 2 below 35 mm. Hg (hypocapnia caused by. hyperventilation, i. e. , increased VA) and a p. H on the alkalotic side (>7. 40) depending on the level of compensation by the kidneys Pa. CO 2 p. H
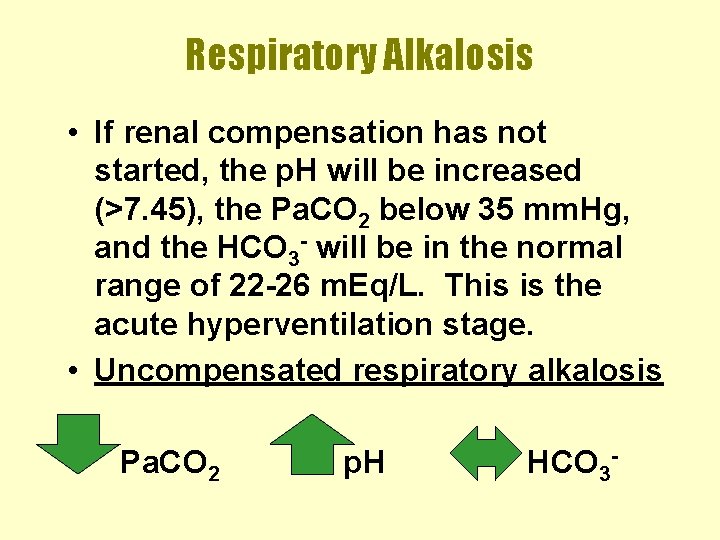
Respiratory Alkalosis • If renal compensation has not started, the p. H will be increased (>7. 45), the Pa. CO 2 below 35 mm. Hg, and the HCO 3 - will be in the normal range of 22 -26 m. Eq/L. This is the acute hyperventilation stage. • Uncompensated respiratory alkalosis Pa. CO 2 p. H HCO 3 -
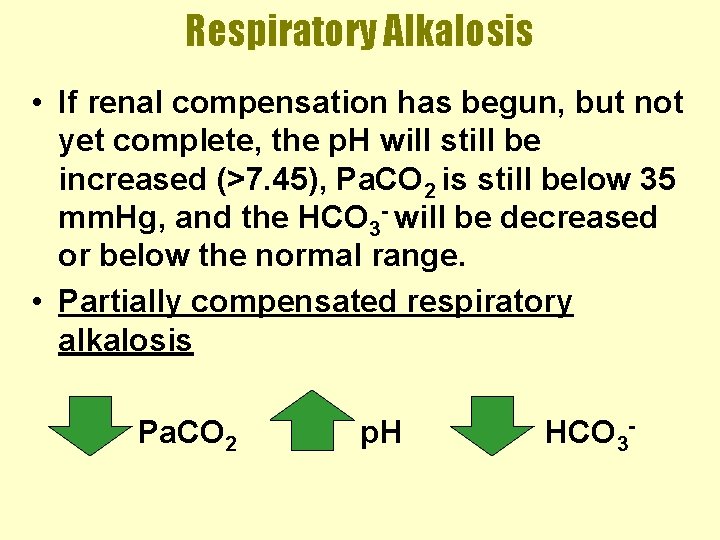
Respiratory Alkalosis • If renal compensation has begun, but not yet complete, the p. H will still be increased (>7. 45), Pa. CO 2 is still below 35 mm. Hg, and the HCO 3 - will be decreased or below the normal range. • Partially compensated respiratory alkalosis Pa. CO 2 p. H HCO 3 -
Respiratory Alkalosis • If renal compensation is fully completed, the p. H will be back within normal range, below 7. 45 but not less than 7. 40 (the kidneys will not overcompensate), and the PCO 2 will still be decreased, and the HCO 3 - will be decreased. This would be the chronic hyperventilation stage. • Fully compensated respiratory alkalosis Pa. CO 2 p. H HCO 3 -

Respiratory Alkalosis • To correct respiratory alkalosis, therapist must remove the cause of the hyperventilation. Ex. Hypoxemia causes respiratory alkalosis, so supplemental oxygen would be indicated.

Common Causes of Respiratory Alkalosis In healthy lungs, other things are increasing. ventilation (VA): • • • Anxiety Pain See Table 7 -3 Fever CNS lesions or brain inflammation Stimulant drugs Hypoxia – several causes

Common Causes of Respiratory Alkalosis Abnormal lungs or conditions: • Hypoxemia • Acute asthma, pneumonia, or pulmonary edema • Diffusion problems • Pulmonary vascular disease • Stimulation of vagal lung receptors which gives the sensation of dyspnea

Common Causes of Respiratory Alkalosis Iatrogenic (caused by medical intervention or treatment) hyperventilation can occur in normal and abnormal lung conditions.

Clinical Manifestations of Respiratory Alkalosis • Tingling in the hands, face or toes • Dizziness • Restlessness and other signs of hypoxemia • Tetany • Coma
Treatment of Respiratory Alkalosis Treat the underlying problem
Metabolic Acidosis Characterized by a decreased bicarbonate HCO 3 - level (<22 m. Eq/L) and a p. H on the acidic side (<7. 40) depending on level of respiratory compensation. HCO 3 - p. H
Metabolic Acidosis • If compensation by the lungs has not begun, the p. H will be less than 7. 35, the HCO 3 - is less than 22 m. Eq/L, and the Pa. CO 2 will be in the normal range of 35 -45 mm. Hg. • Uncompensated metabolic acidosis HCO 3 - p. H Pa. CO 2
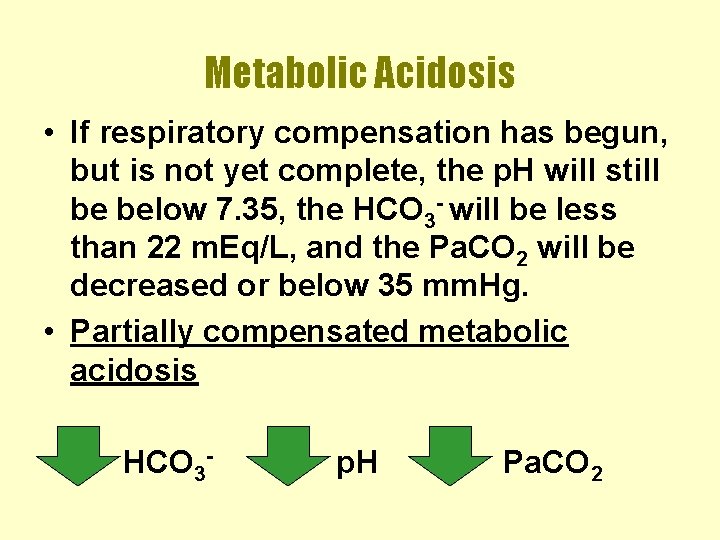
Metabolic Acidosis • If respiratory compensation has begun, but is not yet complete, the p. H will still be below 7. 35, the HCO 3 - will be less than 22 m. Eq/L, and the Pa. CO 2 will be decreased or below 35 mm. Hg. • Partially compensated metabolic acidosis HCO 3 - p. H Pa. CO 2
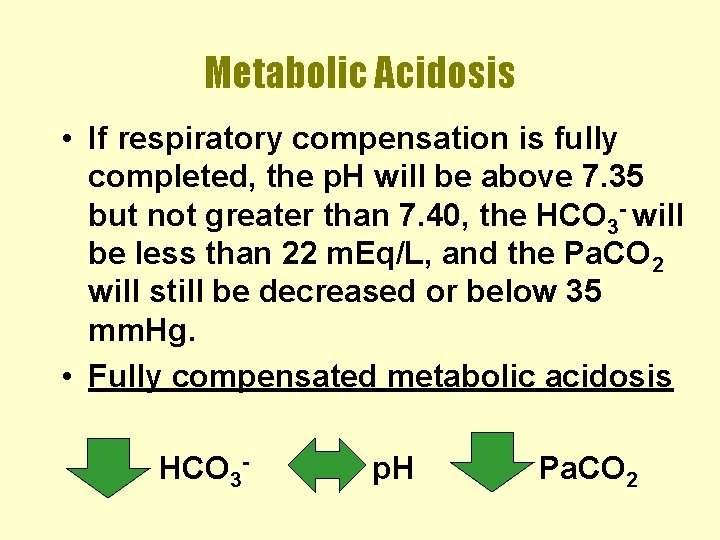
Metabolic Acidosis • If respiratory compensation is fully completed, the p. H will be above 7. 35 but not greater than 7. 40, the HCO 3 - will be less than 22 m. Eq/L, and the Pa. CO 2 will still be decreased or below 35 mm. Hg. • Fully compensated metabolic acidosis HCO 3 - p. H Pa. CO 2

Metabolic Acidosis • Metabolic acidosis can be caused by additional acids in the body that use up the normal levels of HCO 3 - (as a buffer), or the physical loss of the bicarbonate ions or bases from the body. To correct metabolic acidosis, treatment of the underlying cause of acid gain or base loss is the best approach.

Common Causes of Metabolic Acidosis • Causes of metabolic acidosis are often characterized by whether or not there is an increased Anion Gap or a constant/normal in Anion Gap • See Chang, ch. 4 for calculation & explanation
![Anion Gap = Na+ – [Cl- + HCO 3 -] Na+ = 140 m. Anion Gap = Na+ – [Cl- + HCO 3 -] Na+ = 140 m.](http://slidetodoc.com/presentation_image_h2/14caf4d869b45567aa9c20b195e9b65a/image-27.jpg)
Anion Gap = Na+ – [Cl- + HCO 3 -] Na+ = 140 m. Eq/L Cl- = 105 m. Eq/L HCO 3 - = 24 m. Eq/L Normal value is 9 -14 m. Eq/L Rule of thumb: Metabolic acidosis accompanied by a high anion gap means that the body has accumulated an unusual fixed acid. A metabolic acidosis accompanied by a normal anion gap means that the body has lost a greater than normal number of bicarbonate ions. Knowing the patient’s anion gap number aids in identifying appropriate treatment options.
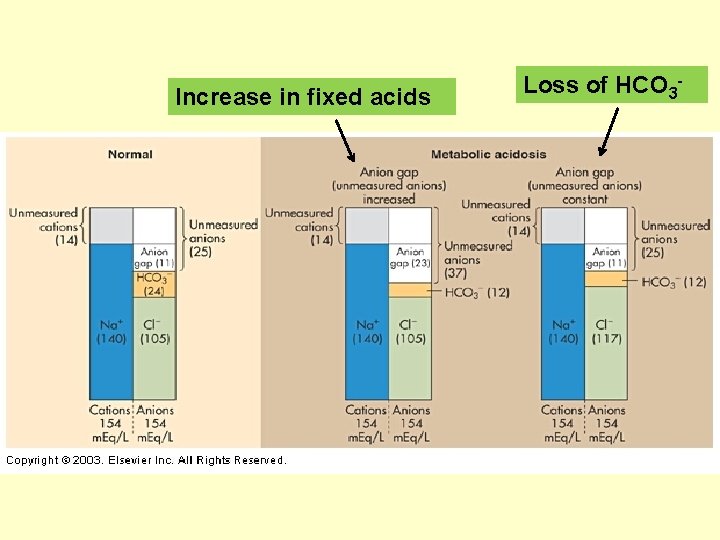
Increase in fixed acids Loss of HCO 3 -

Common Causes of Metabolic Acidosis Increased Anion Gap: • Lactic acidosis See Table 7 -4 • Anaerobic metabolism without O 2 • Ketoacidosis • Diabetes, starvation, alcohol abuse, high fat diet, sometimes in pregnancy • Increased ketones • Renal failure • Increased H+ as it is not excreted in urine
Common Causes of Metabolic Acidosis Increased Anion Gap (cont) • Ingestion of acids • Salicylate intoxication (aspirin) • Methanol or formic acid (formaldehyde) • Ethylene glycol or oxalic acid (antifreeze)
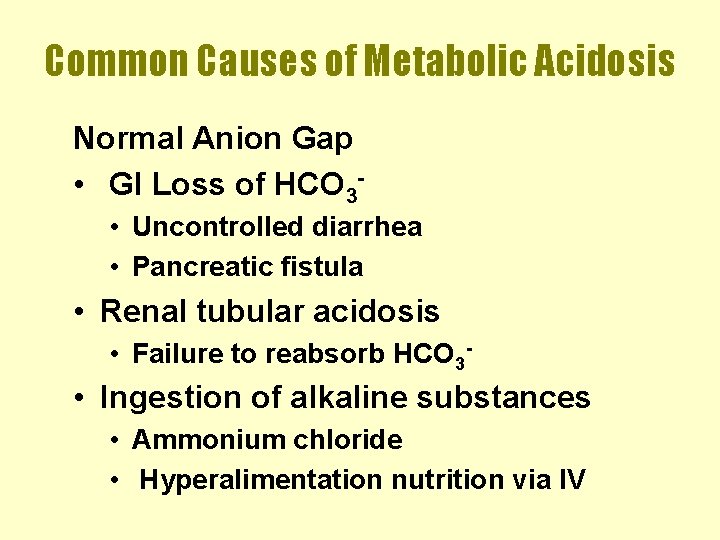
Common Causes of Metabolic Acidosis Normal Anion Gap • GI Loss of HCO 3 • Uncontrolled diarrhea • Pancreatic fistula • Renal tubular acidosis • Failure to reabsorb HCO 3 - • Ingestion of alkaline substances • Ammonium chloride • Hyperalimentation nutrition via IV

Clinical Manifestations of Metabolic Acidosis • Nausea and vomiting • Lethargy – muscle weakness and fatigue • Dysrhythmias – especially with lower p. Hs • Coma

Treatment of Metabolic Acidosis Treat the underlying problem
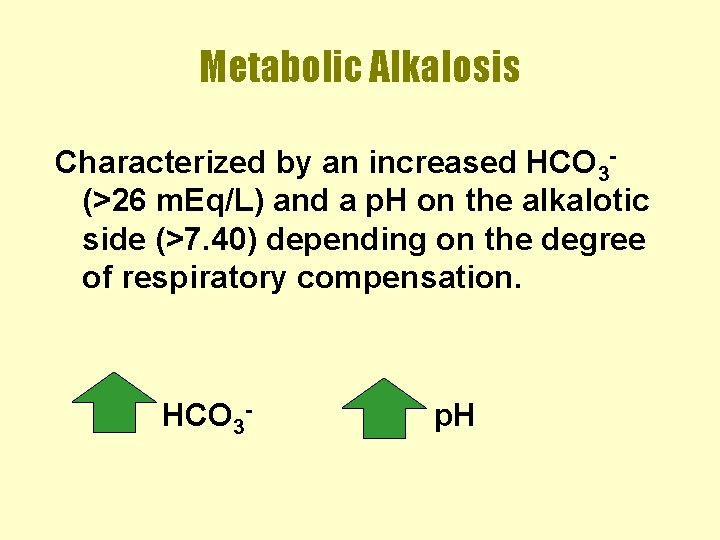
Metabolic Alkalosis Characterized by an increased HCO 3(>26 m. Eq/L) and a p. H on the alkalotic side (>7. 40) depending on the degree of respiratory compensation. HCO 3 - p. H

Metabolic Alkalosis • If compensation by the lungs has not begun, the p. H will be greater than 7. 45, the HCO 3 - is more than 26 m. Eq/L, and the Pa. CO 2 will be in the normal range of 35 -45 mm. Hg. • Uncompensated metabolic alkalosis HCO 3 - p. H Pa. CO 2

Metabolic Alkalosis • If respiratory compensation has begun, but is not yet complete, the p. H will still be above 7. 45, the HCO 3 - will be more than 26 m. Eq/L, and the Pa. CO 2 will be increased or above 45 mm. Hg. • Partially compensated metabolic alkalosis HCO 3 - p. H Pa. CO 2
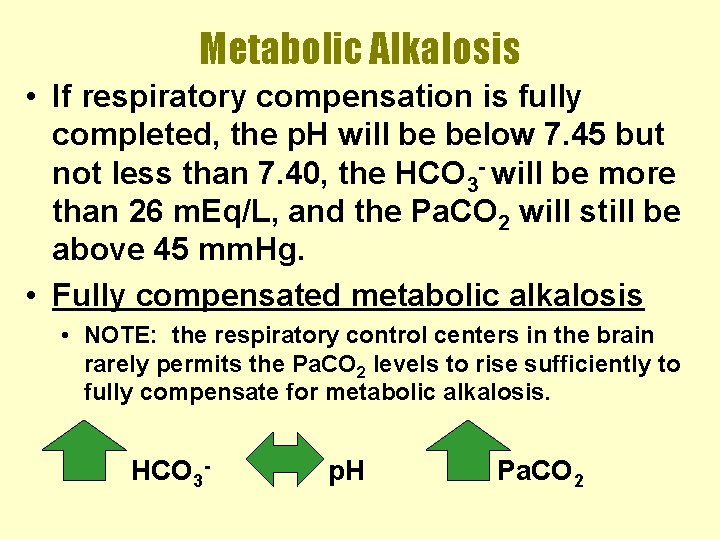
Metabolic Alkalosis • If respiratory compensation is fully completed, the p. H will be below 7. 45 but not less than 7. 40, the HCO 3 - will be more than 26 m. Eq/L, and the Pa. CO 2 will still be above 45 mm. Hg. • Fully compensated metabolic alkalosis • NOTE: the respiratory control centers in the brain rarely permits the Pa. CO 2 levels to rise sufficiently to fully compensate for metabolic alkalosis. HCO 3 - p. H Pa. CO 2

Metabolic Alkalosis • Metabolic alkalosis can be caused by a loss of acids (particularly hydrogen ions – H+) from the body that gives an excess of HCO 3 - (less is needed as a buffer), or a gain of bases or HCO 3 -. To correct metabolic alkalosis, treatment of the underlying cause of acid loss or excess base is recommended. Restoring normal fluid volume and electrolyte concentrations – especially K+ and Cllevels – is also a high priority.

Common Causes of Metabolic Alkalosis Loss of acid (H+) • Excessive vomiting or nasograstric drainage • Diuretics • Hypochloremia (decreased Cl-) • Hypokalemia (decreased K+) • Hypovolemia (decreased fluid volume) • Excessive corticosteriods
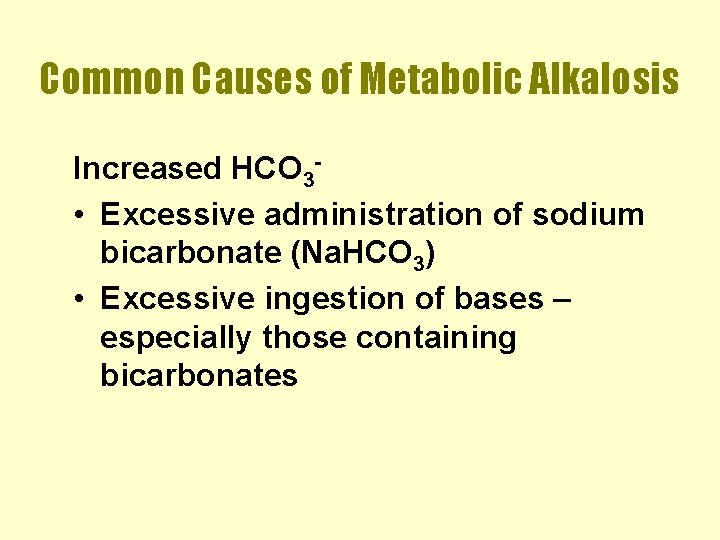
Common Causes of Metabolic Alkalosis Increased HCO 3 • Excessive administration of sodium bicarbonate (Na. HCO 3) • Excessive ingestion of bases – especially those containing bicarbonates

Clinical Manifestations of Metabolic Alkalosis • “Clinical triad” • Loss of Cl- and K+ leads to life threatening conditions • Triple/compounding effects of • Metabolic alkalosis • Muscular weakness (including diaphragm) • Cardiac dysrhythmias • Very common in acutely ill patients and difficult to treat

Treatment of Metabolic Alkalosis Treat the underlying problem
Both (or Combined) Respiratory and Metabolic Acidosis and Alkalosis Combined acid-base imbalances tend to occur in complicated patients in the hospital setting. Typically, the combined states occur due to treatment, or an acute change in the patient’s condition.

Both (or Combined) Respiratory and Metabolic Acidosis and Alkalosis Ex. A patient may be in metabolic acidosis due to ketoacidosis. However, their status changes dramatically when they go into cardiac arrest. The lack of breathing will superimpose a respiratory acidosis on top of the metabolic acidosis to give you the combined state.
- Slides: 44