AcidBase Balance Disturbances Hydrogen ion homeostasis Acids are

Acid-Base Balance Disturbances
Hydrogen ion homeostasis Acids are produced continuously during normal metabolism. (provide H+ to blood) H+ ion concentration of blood varies between narrow limits p. H of the extracellular fluid = 7. 35 – 7. 45 Constant H+ concentration within physiological limits is physiologically important to preserve the enzyme activity and metabolism
Buffering of acids (H+) in blood H+ is generated during intracellular metabolism from several sources (~ 150 000 mmol H+ is produced every day) They are continuously neutralized by buffers resulting in no gain of H+ ions = No p. H change
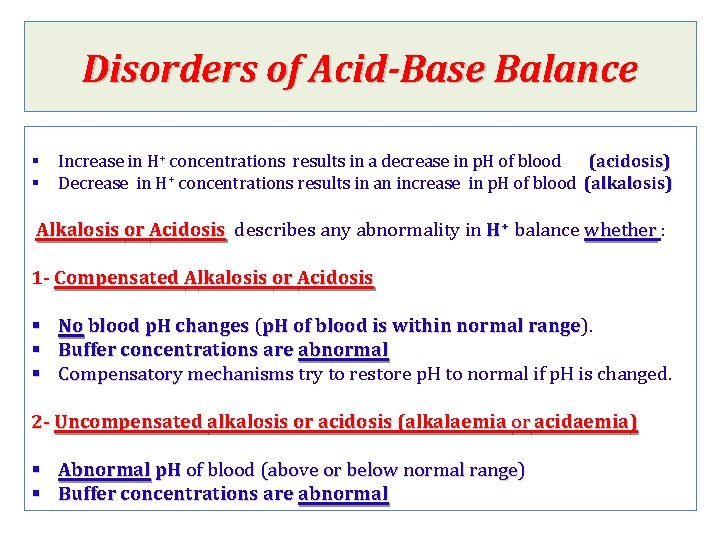
Disorders of Acid-Base Balance § § Increase in H+ concentrations results in a decrease in p. H of blood (acidosis) Decrease in H+ concentrations results in an increase in p. H of blood (alkalosis) Alkalosis or Acidosis describes any abnormality in H+ balance whether : whether 1 - Compensated Alkalosis or Acidosis § No blood p. H changes ( changes p. H of blood is within normal range). range § Buffer concentrations are abnormal § Compensatory mechanisms try to restore p. H to normal if p. H is changed. Compensatory mechanisms 2 - 2 - Uncompensated alkalosis or acidosis (alkalaemia or acidaemia) § Abnormal p. H of blood (above or below normal range) § Buffer concentrations are abnormal
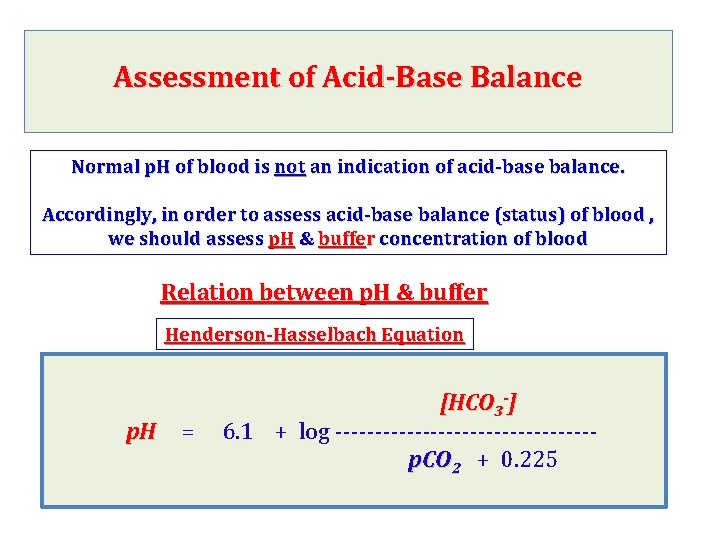
Assessment of Acid-Base Balance Normal p. H of blood is not an indication of acid-base balance. Accordingly, in order to assess acid-base balance (status) of blood , we should assess p. H & buffer concentration of blood Relation between p. H & buffer Henderson-Hasselbach Equation [HCO 3 -] p. H = 6. 1 + log ---------------- p. CO 2 + 0. 225

Acid-Base Balance Disturbances Acid-base 1 - Acidosis : - Metabolic ↓↓ HCO 3 - Respiratory ↑↑ CO 2 2 - Alkalosis : - Metabolic ↑↑ HCO 3 - Respiratory ↓↓ CO 2 DIAGNOSIS IS CONFIRMED BY LABORATORY INVESTIGATIONS OF p. H, p. CO 2 & p. O 2 & HCO 3 Sample: Arterial Blood using Procedure: Blood gas analysis
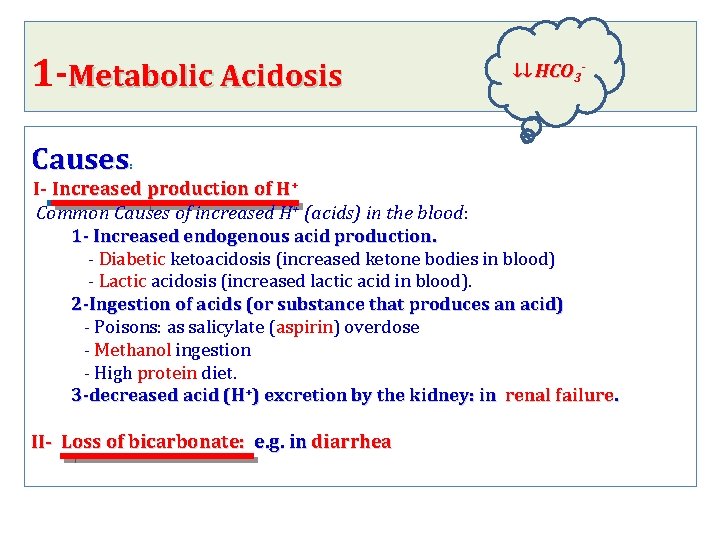
1 -Metabolic Acidosis ↓↓ HCO 3 - Causes : I- Increased production of H+ Common Causes of increased H+ (acids) in the blood: 1 - Increased endogenous acid production. - Diabetic ketoacidosis (increased ketone bodies in blood) - Lactic acidosis (increased lactic acid in blood). 2 -Ingestion of acids (or substance that produces an acid) - Poisons: as salicylate (aspirin) overdose - Methanol ingestion - High protein diet. 3 -decreased acid (H+) excretion by the kidney: in renal failure. II- Loss of bicarbonate: e. g. in diarrhea

Metabolic Acidosis cont Compensatory mechanisms of metabolic acidosis 1 - Exhaustion of bicarbonate buffer with shift of reactions to CO 2 production. Stimulation of the respiratory centre to eliminate excess CO 2 formed (CO 2 wash) 2 - Increase in renal acid excretion of H+

LABORTORY INVESTIGATION: Sample Arterial Blood Equipment: Equipment Blood Gas Analyzer p. H : Low HCO 3: Low PCO 2 : Low: as : Low: CO 2 is produced then exhaled by lungs by rapid respiration PO 2: Normal
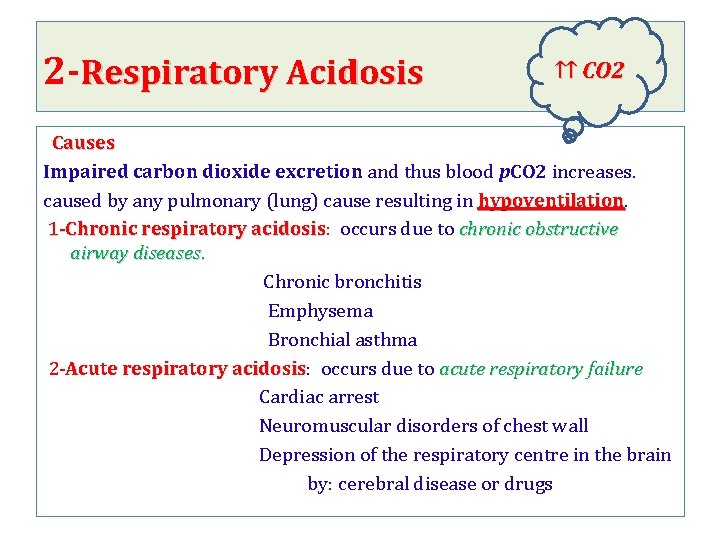
2 -Respiratory Acidosis ↑↑ CO 2 Causes Impaired carbon dioxide excretion and thus blood p. CO 2 increases. caused by any pulmonary (lung) cause resulting in hypoventilation 1 -Chronic respiratory acidosis: occurs due to chronic obstructive respiratory acidosis airway diseases. Chronic bronchitis Emphysema Bronchial asthma 2 -Acute respiratory acidosis: occurs due to acute respiratory failure respiratory acidosis Cardiac arrest Neuromuscular disorders of chest wall Depression of the respiratory centre in the brain by: cerebral disease or drugs
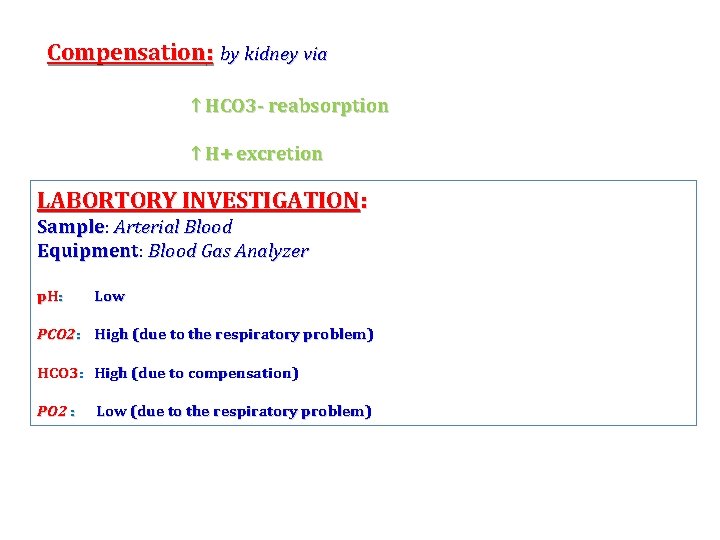
Compensation: by kidney via ↑ HCO 3 - reabsorption ↑ H+ excretion LABORTORY INVESTIGATION: Sample Arterial Blood Equipment: Equipment Blood Gas Analyzer p. H: Low PCO 2: High (due to the respiratory problem) HCO 3: High (due to compensation) PO 2 : Low (due to the respiratory problem)
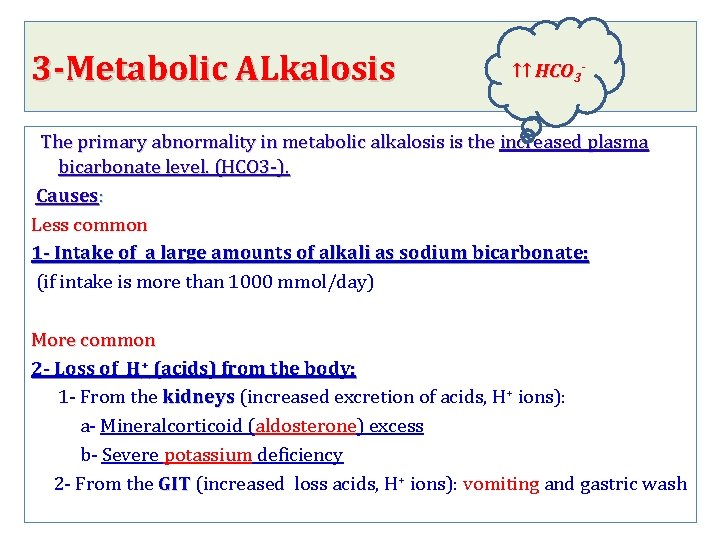
3 -Metabolic ALkalosis ↑↑ HCO 3 - The primary abnormality in metabolic alkalosis is the increased plasma bicarbonate level. (HCO 3 -). Causes: Less common 1 - Intake of a large amounts of alkali as sodium bicarbonate: (if intake is more than 1000 mmol/day) More common 2 - Loss of H+ (acids) from the body: + ions): 1 - From the kidneys (increased excretion of acids, H kidneys a- Mineralcorticoid (aldosterone) excess b- Severe potassium deficiency 2 - From the GIT (increased loss acids, H+ ions): vomiting and gastric wash
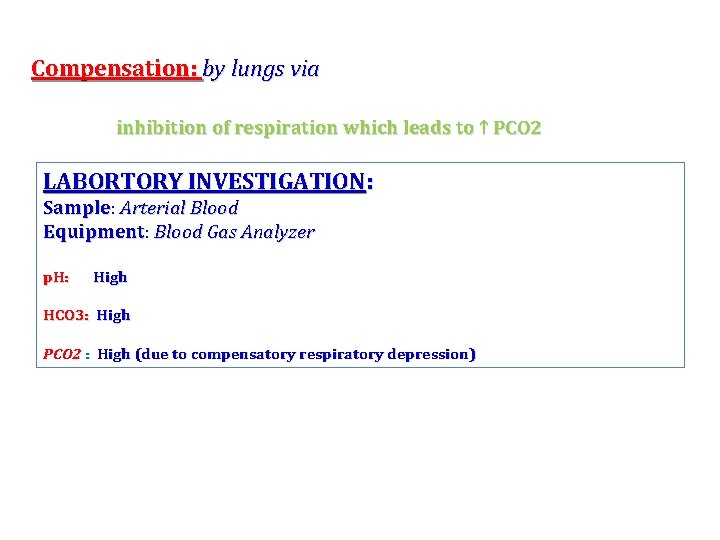
Compensation: by lungs via inhibition of respiration which leads to ↑ PCO 2 LABORTORY INVESTIGATION: Sample Arterial Blood Equipment: Equipment Blood Gas Analyzer p. H: High HCO 3: High PCO 2 : High (due to compensatory respiratory depression)
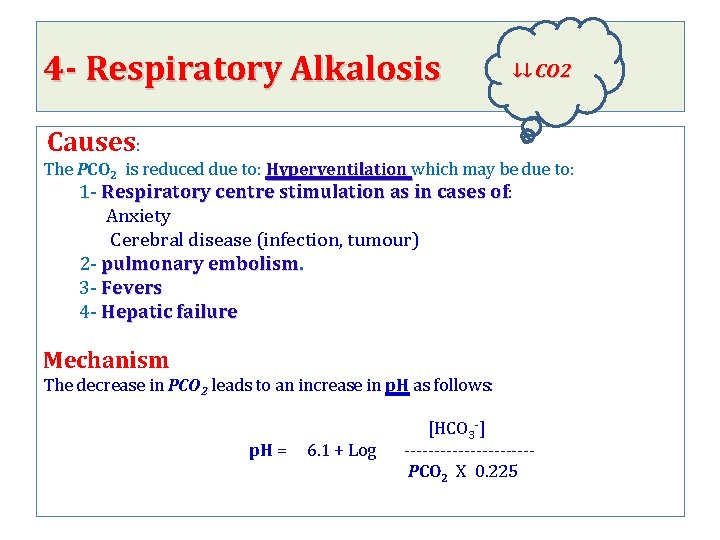
4 - Respiratory Alkalosis ↓↓ CO 2 Causes: The PCO 2 is reduced due to: Hyperventilation which may be due to: 1 - Respiratory centre stimulation as in cases of: stimulation as in cases of Anxiety Cerebral disease (infection, tumour) 2 - pulmonary embolism. 3 - Fevers 4 - Hepatic failure Mechanism The decrease in PCO 2 leads to an increase in p. H as follows: [HCO 3 -] p. H = 6. 1 + Log ----------- PCO 2 X 0. 225

Respiratory Alkalosis Compensation : by kidney by ↓ HCO 3 reabsorption and ↓ H+ secretion LABORTORY INVESTIGATION: Sample Arterial Blood Equipment: Equipment Blood Gas Analyzer p. H: High HCO 3: Low PCO 2 : Low

Practice Problems Acid-Base Imbalances interpretation of Arterial Blood Gases (ABG) RESP
Getting an arterial blood gas sample
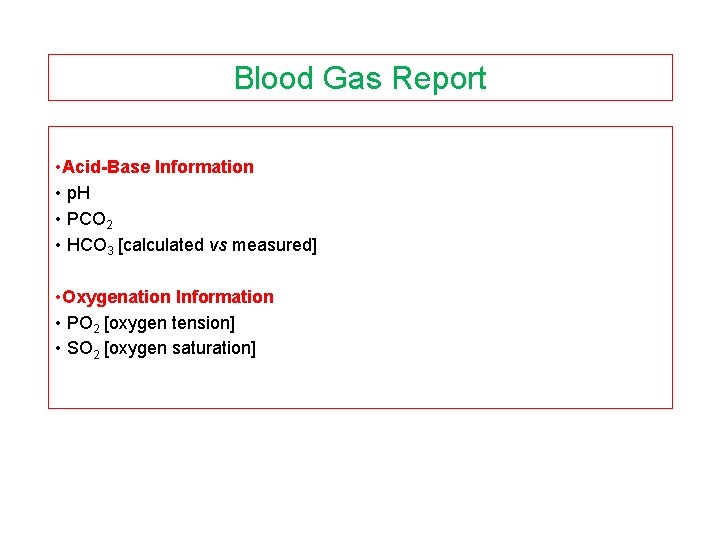
Blood Gas Report • Acid-Base Information • p. H • PCO 2 • HCO 3 [calculated vs measured] • Oxygenation Information • PO 2 [oxygen tension] • SO 2 [oxygen saturation]
![PRIMARY AND SECONDARY ACID-BASE DERANGEMENTS p. H = [HCO 3 -] 6. 1 + PRIMARY AND SECONDARY ACID-BASE DERANGEMENTS p. H = [HCO 3 -] 6. 1 +](http://slidetodoc.com/presentation_image/af34bdb0961a5d882d3df4becca397e0/image-19.jpg)
PRIMARY AND SECONDARY ACID-BASE DERANGEMENTS p. H = [HCO 3 -] 6. 1 + log ----------------PCO 2 + 0. 225 Acid-Base Disorder Primary Change Respiratory acidosis Respiratory alkalosis Metabolic acidosis Metabolic alkalosis PCO 2 up PCO 2 down HCO 3 up Compensatory Change HCO 3 up HCO 3 down PCO 2 up
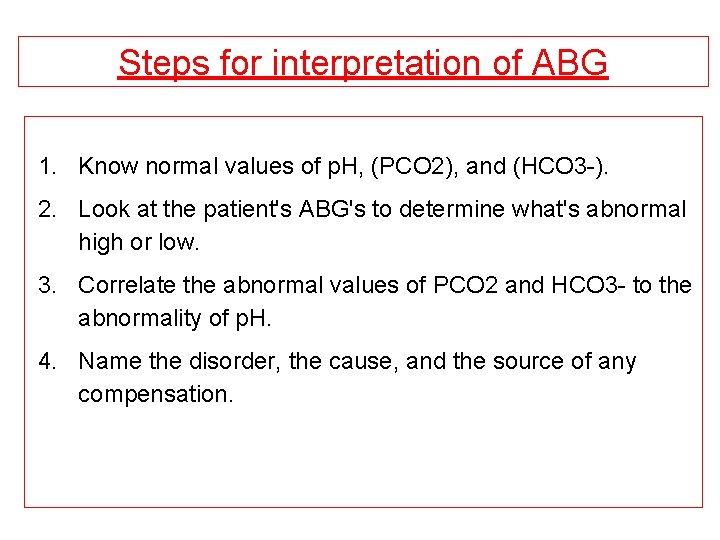
Steps for interpretation of ABG 1. Know normal values of p. H, (PCO 2), and (HCO 3 -). 2. Look at the patient's ABG's to determine what's abnormal high or low. 3. Correlate the abnormal values of PCO 2 and HCO 3 - to the abnormality of p. H. 4. Name the disorder, the cause, and the source of any compensation.

1 - Normal values for ABG's: 2 - Evaluate the patient's ABG's: • is the p. H normal? Is it too high or too low? Is it acidosis or alkalosis? • Is the HCO 3 - normal? Is it too high or too low? Will it cause acidosis or alkalosis? Will it correct acidosis or alkalosis? • Is the CO 2 normal? Is it too high or too low? Will it cause acidosis or alkalosis? Will it correct acidosis or alkalosis?
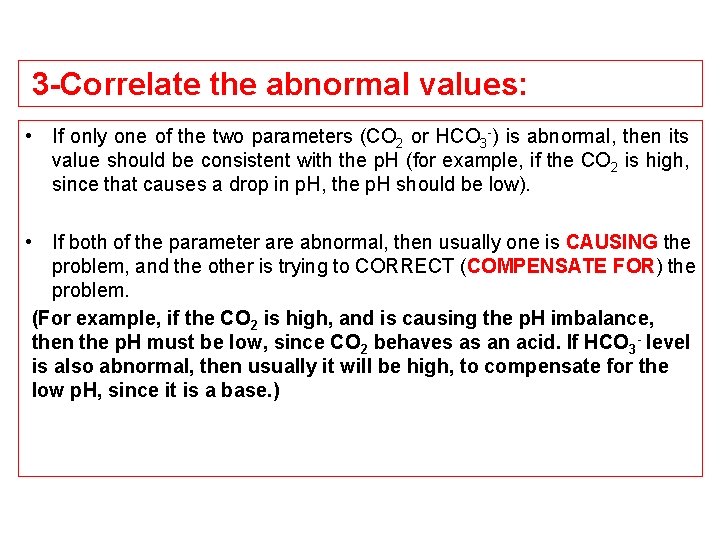
3 -Correlate the abnormal values: • If only one of the two parameters (CO 2 or HCO 3 -) is abnormal, then its value should be consistent with the p. H (for example, if the CO 2 is high, since that causes a drop in p. H, the p. H should be low). • If both of the parameter are abnormal, then usually one is CAUSING the problem, and the other is trying to CORRECT (COMPENSATE FOR) the problem. (For example, if the CO 2 is high, and is causing the p. H imbalance, then the p. H must be low, since CO 2 behaves as an acid. If HCO 3 - level is also abnormal, then usually it will be high, to compensate for the low p. H, since it is a base. )

4 - Name the disorder: Respiratory acidosis (with or without renal compensation) • Respiratory alkalosis(with or without renal compensation) • Metabolic acidosis (with or without respiratory compensation) • Metabolic alkalosis (with or without respiratory compensation) 5 - Suggest a possible cause For example, a cause of chronic respiratory acidosis is emphysema.
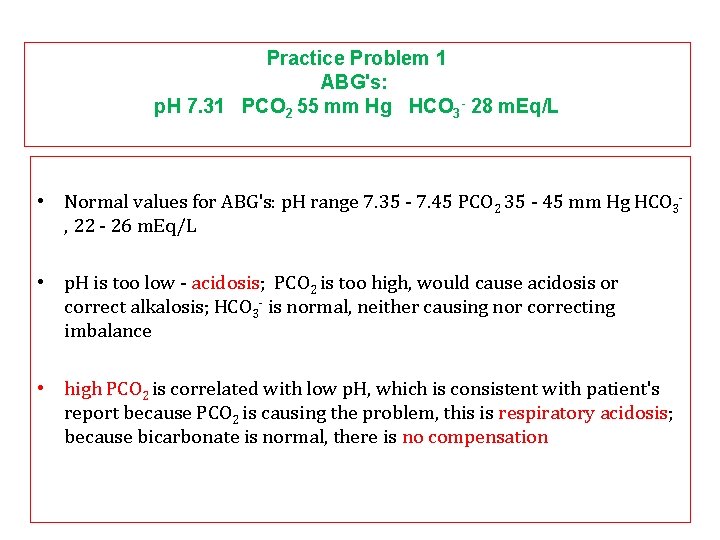
Practice Problem 1 ABG's: p. H 7. 31 PCO 2 55 mm Hg HCO 3 - 28 m. Eq/L • Normal values for ABG's: p. H range 7. 35 - 7. 45 PCO 2 35 - 45 mm Hg HCO 3, 22 - 26 m. Eq/L • p. H is too low - acidosis; PCO 2 is too high, would cause acidosis or correct alkalosis; HCO 3 - is normal, neither causing nor correcting imbalance • high PCO 2 is correlated with low p. H, which is consistent with patient's report because PCO 2 is causing the problem, this is respiratory acidosis; because bicarbonate is normal, there is no compensation
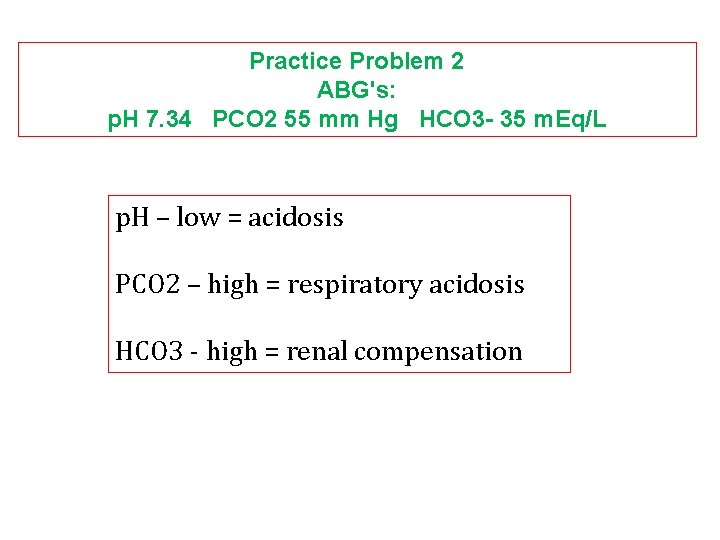
Practice Problem 2 ABG's: p. H 7. 34 PCO 2 55 mm Hg HCO 3 - 35 m. Eq/L p. H – low = acidosis PCO 2 – high = respiratory acidosis HCO 3 - high = renal compensation
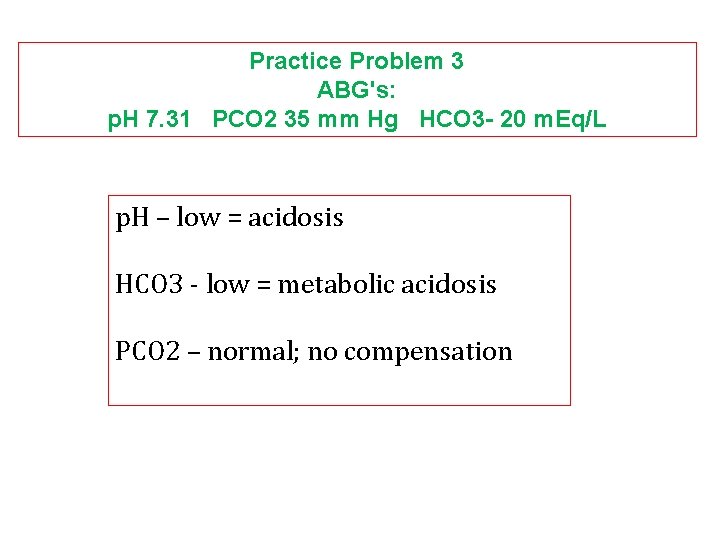
Practice Problem 3 ABG's: p. H 7. 31 PCO 2 35 mm Hg HCO 3 - 20 m. Eq/L p. H – low = acidosis HCO 3 - low = metabolic acidosis PCO 2 – normal; no compensation
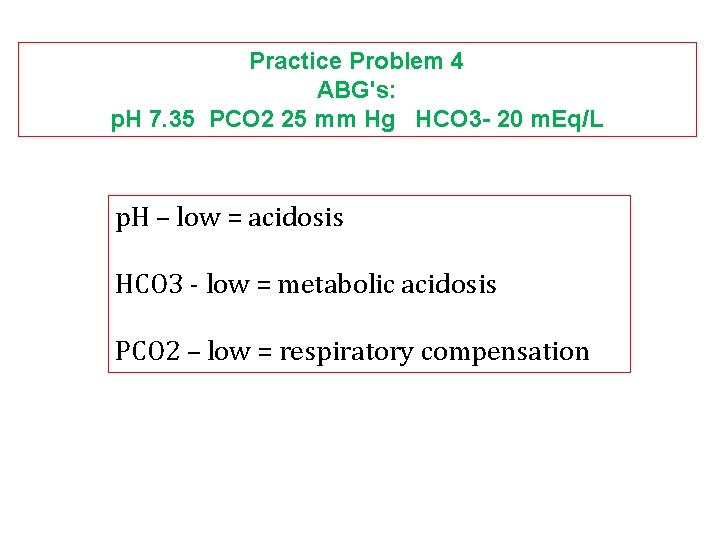
Practice Problem 4 ABG's: p. H 7. 35 PCO 2 25 mm Hg HCO 3 - 20 m. Eq/L p. H – low = acidosis HCO 3 - low = metabolic acidosis PCO 2 – low = respiratory compensation

Practice Problem 5 ABG's: p. H 7. 48 PCO 2 25 mm Hg HCO 3 - 24 m. Eq/L p. H – high = alkalosis PCO 2 – low = respiratory alkalosis HCO 3 - normal; no compensation

Practice Problem 6 ABG's: p. H 7. 44 PCO 2 25 mm Hg HCO 3 - 20 m. Eq/L p. H – high = alkalosis PCO 2 – low = respiratory alkalosis HCO 3 - low = renal compensation

Practice Problem 7 ABG's: p. H 7. 48 PCO 2 40 mm Hg HCO 3 - 33 m. Eq/L p. H – high = alkalosis HCO 3 – high = metabolic alkalosis PCO 2 – normal; no compensation
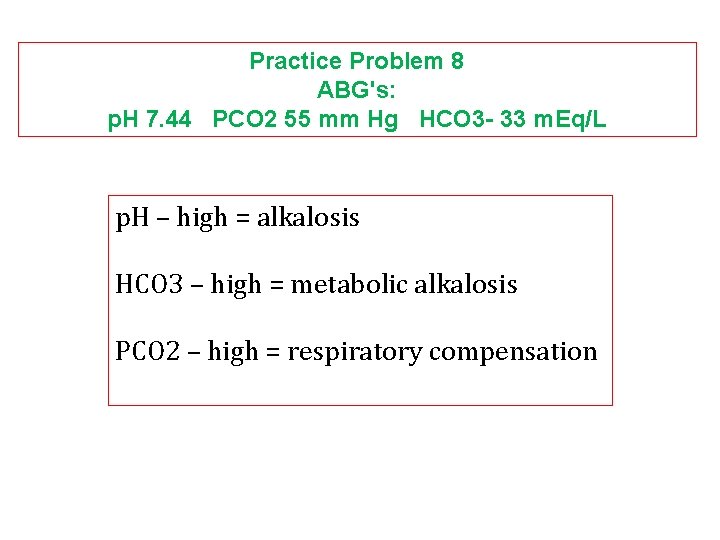
Practice Problem 8 ABG's: p. H 7. 44 PCO 2 55 mm Hg HCO 3 - 33 m. Eq/L p. H – high = alkalosis HCO 3 – high = metabolic alkalosis PCO 2 – high = respiratory compensation
- Slides: 31