AcidBase Arrhenius definition acid base produces H in
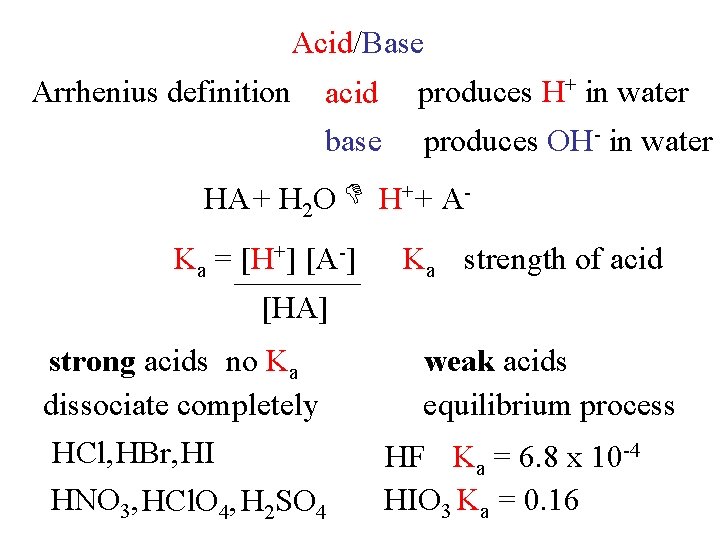
Acid/Base Arrhenius definition acid base produces H+ in water produces OH- in water H+ + A HA + H 2 O Ka = [H+] [A-] [HA] [H 2 O] [HA] strong acids no Ka dissociate completely HCl, HBr, HI HNO 3, HCl. O 4, H 2 SO 4 Ka strength of acid weak acids equilibrium process HF Ka = 6. 8 x 10 -4 HIO 3 Ka = 0. 16
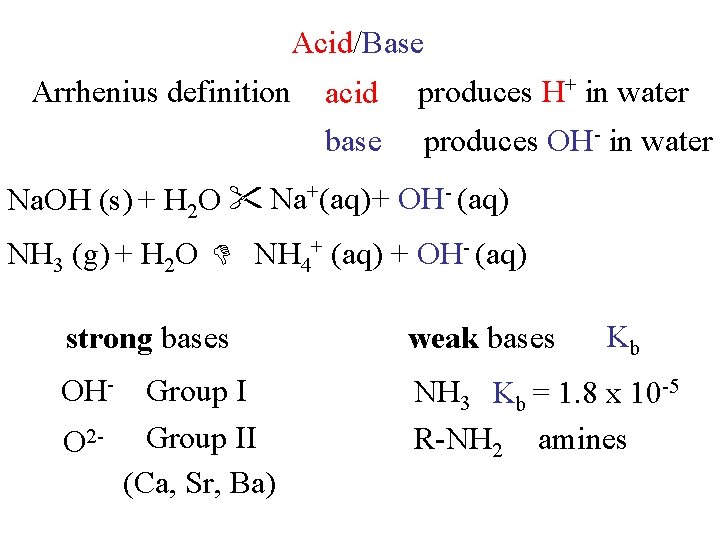
Acid/Base Arrhenius definition acid base produces H+ in water produces OH- in water Na. OH (s) + H 2 O Na+(aq) + OH- (aq) NH 3 (g) + H 2 O NH 4+ (aq) + OH- (aq) Kb strong bases weak bases OH- Group I O 2 - Group II (Ca, Sr, Ba) NH 3 Kb = 1. 8 x 10 -5 R-NH 2 amines
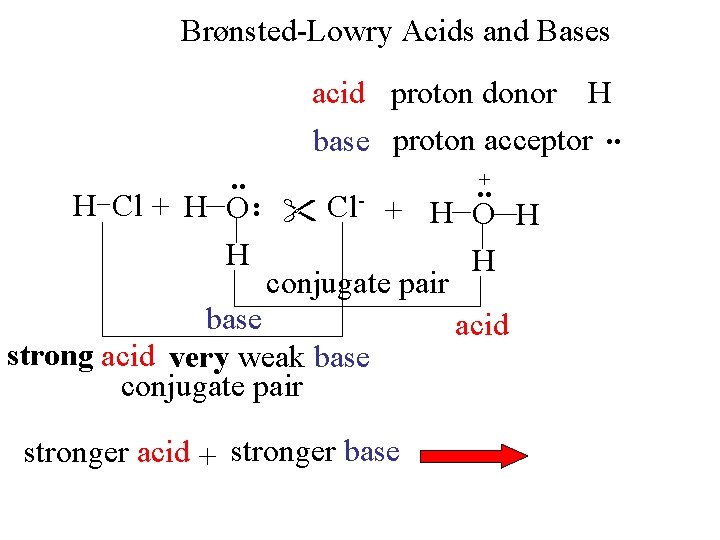
Brønsted-Lowry Acids and Bases . . acid proton donor H base proton acceptor. . +. . H Cl + H O Cl- + H O H H H conjugate pair base acid strong acid very weak base conjugate pair stronger acid + stronger base
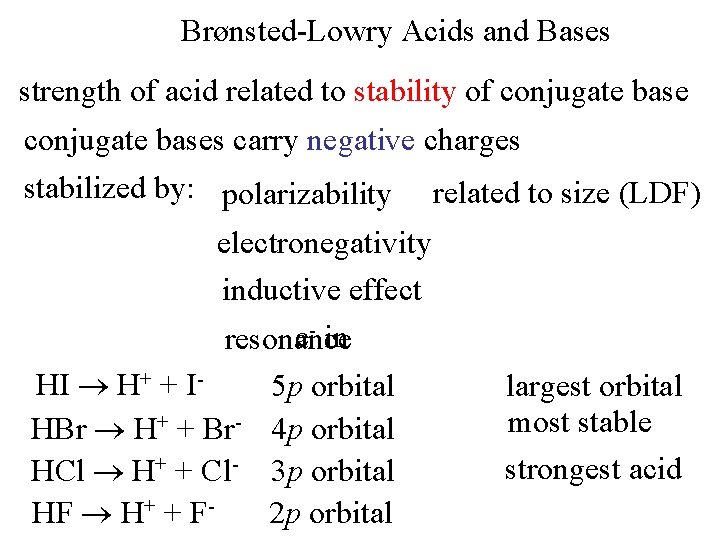
Brønsted-Lowry Acids and Bases strength of acid related to stability of conjugate bases carry negative charges stabilized by: polarizability related to size (LDF) electronegativity inductive effect e- in resonance HI H+ + IHBr H+ + Br. HCl H+ + Cl. HF H+ + F- 5 p orbital 4 p orbital 3 p orbital 2 p orbital largest orbital most stable strongest acid
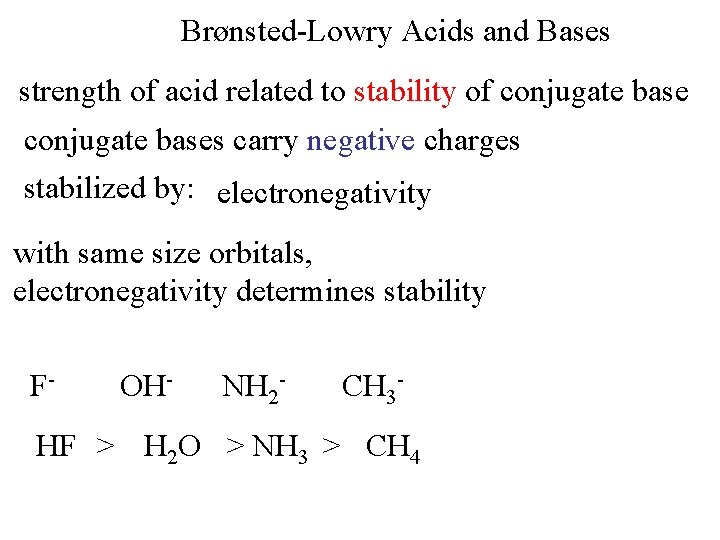
Brønsted-Lowry Acids and Bases strength of acid related to stability of conjugate bases carry negative charges stabilized by: electronegativity with same size orbitals, electronegativity determines stability F- OH- NH 2 - CH 3 - HF > H 2 O > NH 3 > CH 4
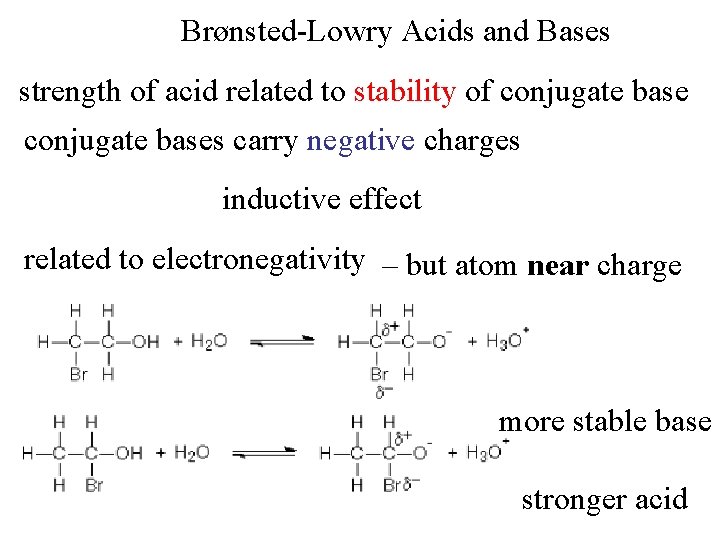
Brønsted-Lowry Acids and Bases strength of acid related to stability of conjugate bases carry negative charges inductive effect related to electronegativity – but atom near charge more stable base stronger acid
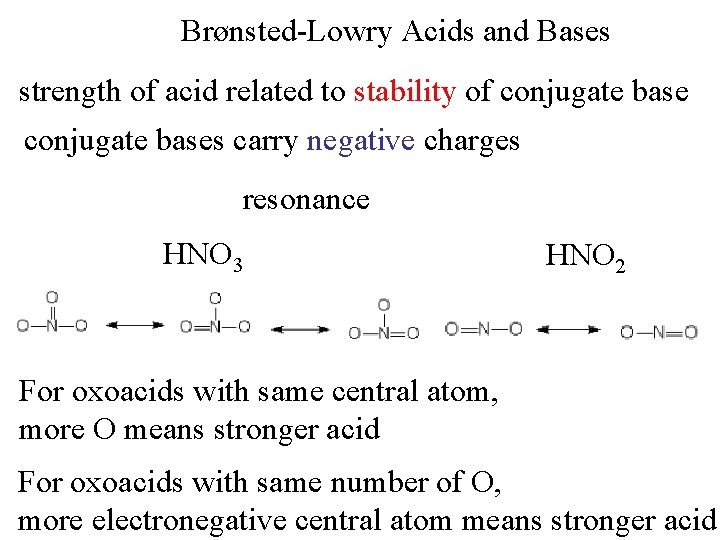
Brønsted-Lowry Acids and Bases strength of acid related to stability of conjugate bases carry negative charges resonance HNO 3 HNO 2 For oxoacids with same central atom, more O means stronger acid For oxoacids with same number of O, more electronegative central atom means stronger acid
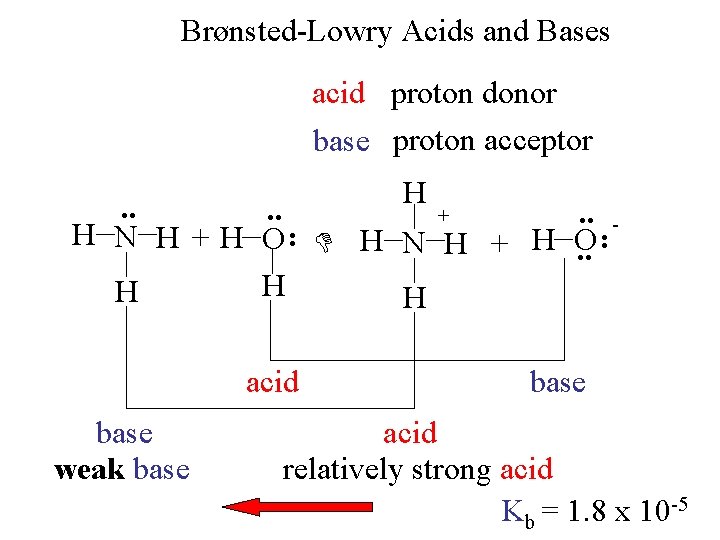
Brønsted-Lowry Acids and Bases acid proton donor base proton acceptor H acid base weak base - . . + H N H +H O H N H + H O. . H H H base acid relatively strong acid Kb = 1. 8 x 10 -5
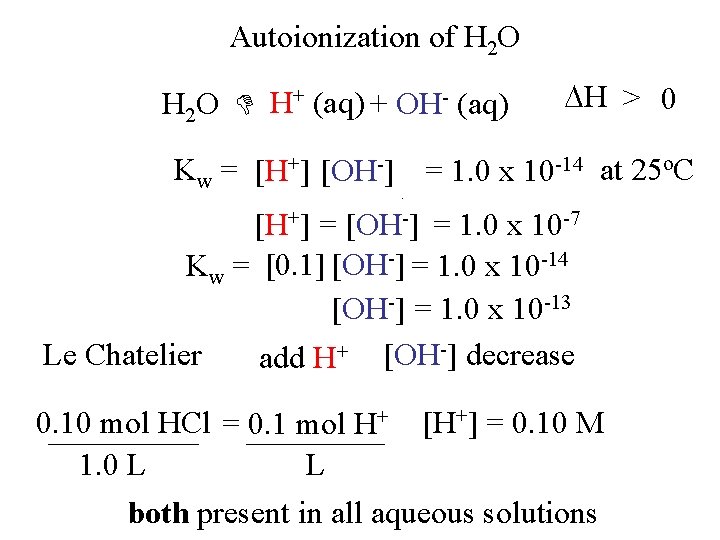
Autoionization of H 2 O H+ (aq) + OH- (aq) H > 0 Kw = [H+] [OH-] = 1. 0 x 10 -14 at 25 o. C +] =O] -] 55. 5 -7 M [H[H [OH = 1. 0 x 10 2 0. 0000001 Kw = [0. 1] [OH-] =-1. 0 x 10 -14 [OH-] = 1. 0 x 10 -13 -] decrease + Le Chatelier [OH add H 0. 10 mol HCl = 0. 1 mol H+ [H+] = 0. 10 M 1. 0 L L both present in all aqueous solutions
![p. H [H+] 10 M - 10 -15 M low p. H -1 acidic p. H [H+] 10 M - 10 -15 M low p. H -1 acidic](http://slidetodoc.com/presentation_image_h2/53c90e07565e7b4d367fd6dc3dd80c9e/image-10.jpg)
p. H [H+] 10 M - 10 -15 M low p. H -1 acidic p. H = - log [H+] 15 high p. H basic neutral [H+] = [OH-] = 1. 0 x 10 -7 p. H = 7 p. H + p. OH = 14 p. OH = - log [OH-] p. H of milk = 6. 4 [H+] = 3. 981071706 x 10 -7 10 -6. 4 4 x 10 -7 1 sig. fig.
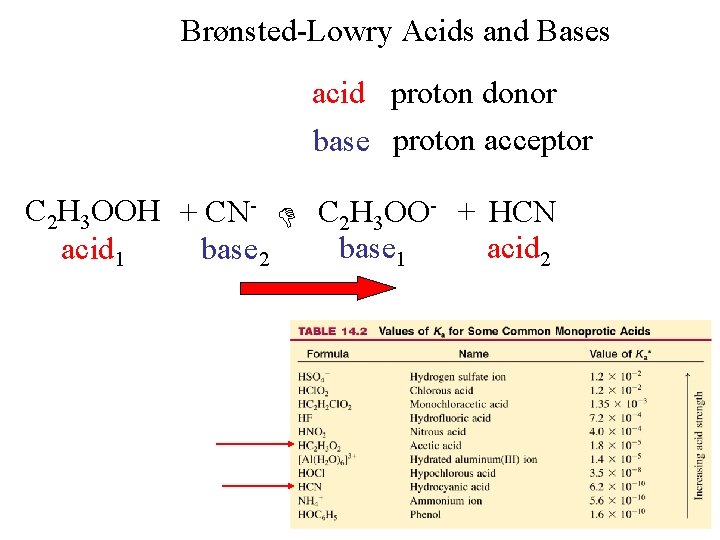
Brønsted-Lowry Acids and Bases acid proton donor base proton acceptor C 2 H 3 OOH + CN- C 2 H 3 OO- + HCN base 1 acid 2 acid 1 base 2
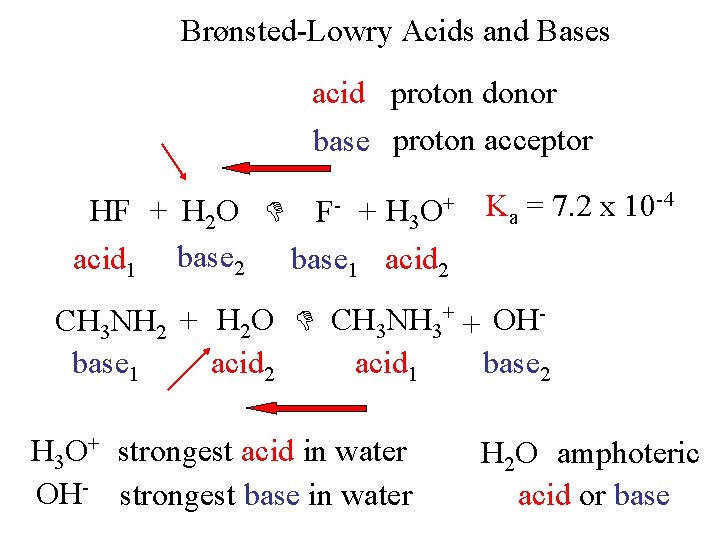
Brønsted-Lowry Acids and Bases acid proton donor base proton acceptor HF + H 2 O + acid 1 base 2 base 1 acid 2 F- H 3 O+ Ka = 7. 2 x 10 -4 CH 3 NH 2 + H 2 O CH 3 NH 3+ + OHbase 1 acid 2 acid 1 base 2 H 3 O+ strongest acid in water OH- strongest base in water H 2 O amphoteric acid or base
- Slides: 12