Acid Naming Rules Is it an acid All













- Slides: 14

Acid Naming Rules

Is it an acid? • All acids that we will work with donate hydrogen ions [H+] in water, (or we say they are proton donors. ) • To do this they have to have a hydrogen as the main cation. • Examples HNO 3 and HCl

Decide if it is Binary or Ternary • Binary are two element compounds like HCl and HBr. • Ternary are three element compounds like HNO 3 and H 2 SO 4

If it is Binary • Find the root such as in HCl the root is “chlor” • Use the prefix HYDRO • Add the root • And change the suffix of the anion from ide to ic. • Result: Hydrochloric acid • Binary acid formula: Hydro-root-ic acid

If it is Ternary • Drop the hydrogen and name the anion. • Change suffix “ate” to “ic” and “ite” to “ous” • Examples: H 2 SO 4 Hydrogen sulfate becomes Sulfuric acid H 2 SO 3 Hydrogen sulfite becomes Sulfurous acid

Is it that easy? Yes

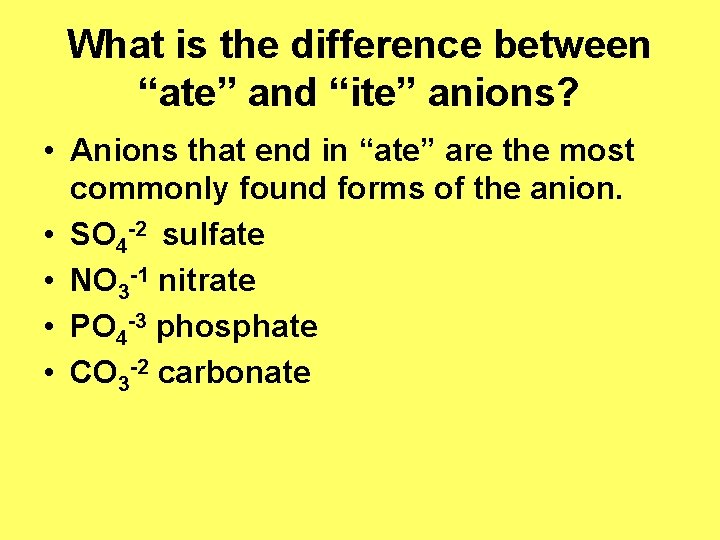
What is the difference between “ate” and “ite” anions? • Anions that end in “ate” are the most commonly found forms of the anion. • SO 4 -2 sulfate • NO 3 -1 nitrate • PO 4 -3 phosphate • CO 3 -2 carbonate

“ite” means one less oxygen that the “ate” form • SO 3 -2 sulfite SO 4 -2 sulfate • NO 2 -1 nitrite NO 3 -1 nitrate • PO 3 -3 phosphite PO 4 -3 phosphate • CO 2 -2 carbonite CO 3 -2 carbonate

Find the back of your oxidation chart • Note all the bold faced anions going down the center of the page. • Those are all the “ate” ions or polyatomic ions that are designated the most common form. • Find Cl. O 3 -1 (chlorate) • Find chlorite • Cl. O 2 -1 (chlorite)

Look up the names of the following ions: • Look in the upper right hand side of your oxidation chart • Cl. O-1 • hypochlorite • Cl. O 4 -1 • perchlorate

The prefix “hypo” • Hypo means…. below or under as in hypodermic needle, below the skin, or hypo glycemic-low blood sugar. • Cl. O-1 • Hypochlorite- one less oxygen than chlorite

The prefix “hyper” or “per” • Hyper means more than or above as in hyperactive, or hyperglycemic- too much blood sugar. • Chemists drop the “hy” and just use “per. ” • Cl. O 4 -1 • perchlorate

Practice naming acids • • How would you name HCl. O 3? Chloric acid How would you name HCl. O 2? Chlorous acid How would you name HCl. O? Hypochlorous acid How would you name HCl. O 4? Perchloric acid

Practice naming sodium salts • • How would you name Na. Cl. O 3? Sodium chlorate How would you name Na. Cl. O 2? Sodium chlorite How would you name Na. Cl. O? Sodium hypochlorite How would you name Na. Cl. O 4? Sodium perchlorate