Acid Mine Water Can it be a valuable

Acid Mine Water – Can it be a valuable resource?

Waterfall, Witwatersrand National Botanical Gardens

The farm where gold was first discovered in 1886 by that an Australian gold miner, George Harrison
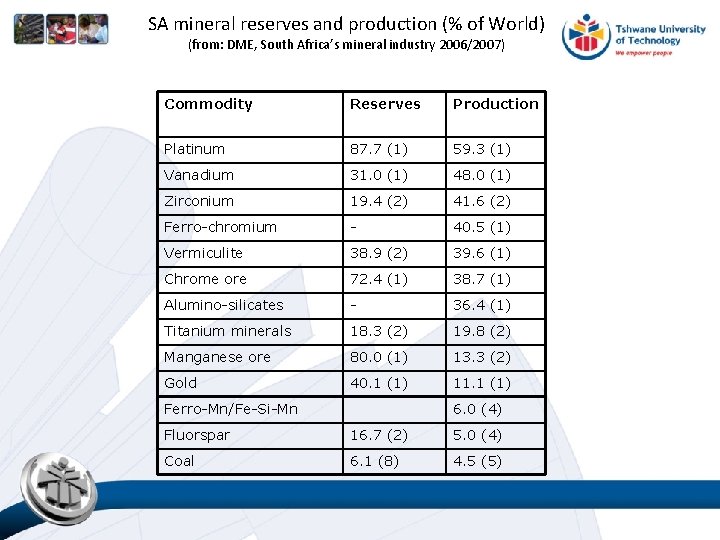
SA mineral reserves and production (% of World) (from: DME, South Africa’s mineral industry 2006/2007) Commodity Reserves Production Platinum 87. 7 (1) 59. 3 (1) Vanadium 31. 0 (1) 48. 0 (1) Zirconium 19. 4 (2) 41. 6 (2) Ferro-chromium - 40. 5 (1) Vermiculite 38. 9 (2) 39. 6 (1) Chrome ore 72. 4 (1) 38. 7 (1) Alumino-silicates - 36. 4 (1) Titanium minerals 18. 3 (2) 19. 8 (2) Manganese ore 80. 0 (1) 13. 3 (2) Gold 40. 1 (1) 11. 1 (1) Ferro-Mn/Fe-Si-Mn 6. 0 (4) Fluorspar 16. 7 (2) 5. 0 (4) Coal 6. 1 (8) 4. 5 (5)
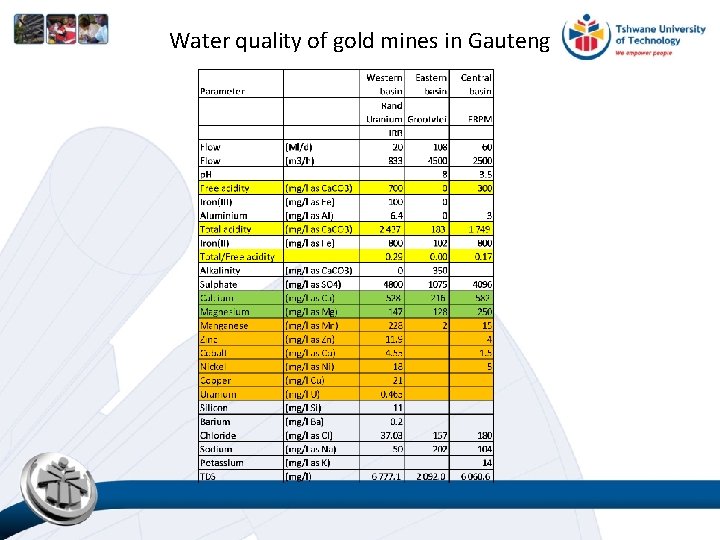
Water quality of gold mines in Gauteng
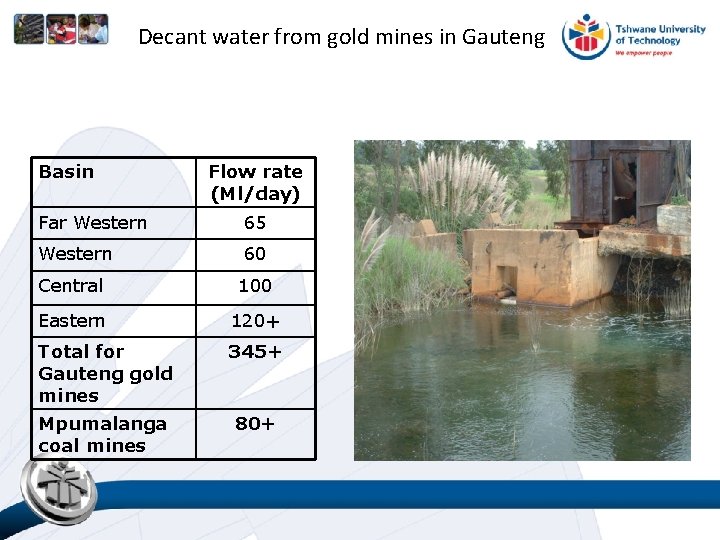
Decant water from gold mines in Gauteng Basin Flow rate (Ml/day) Far Western 65 Western 60 Central 100 Eastern 120+ Total for Gauteng gold mines 345+ Mpumalanga coal mines 80+
Limited area
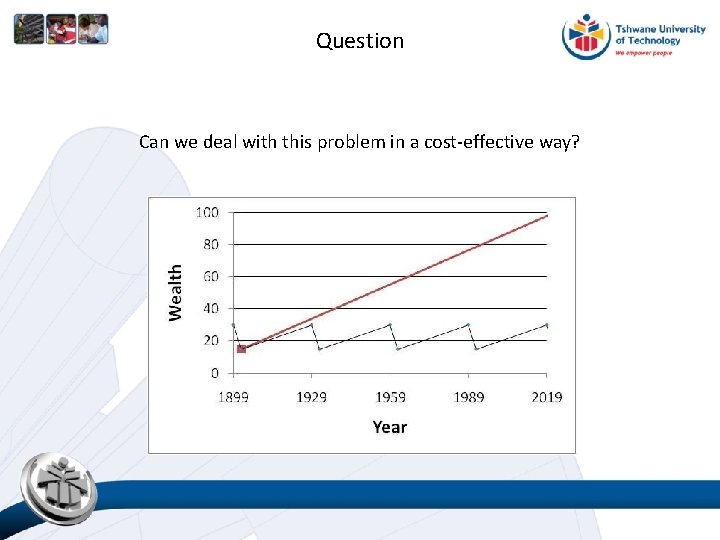
Question Can we deal with this problem in a cost-effective way?

Neutralized mine water
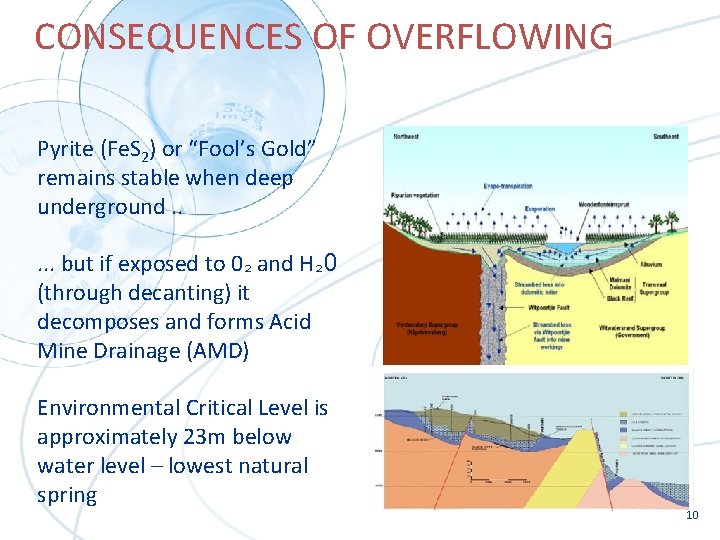
CONSEQUENCES OF OVERFLOWING Pyrite (Fe. S 2) or “Fool’s Gold” remains stable when deep underground. . . but if exposed to 0² and H² 0 (through decanting) it decomposes and forms Acid Mine Drainage (AMD) Environmental Critical Level is approximately 23 m below water level – lowest natural spring 10
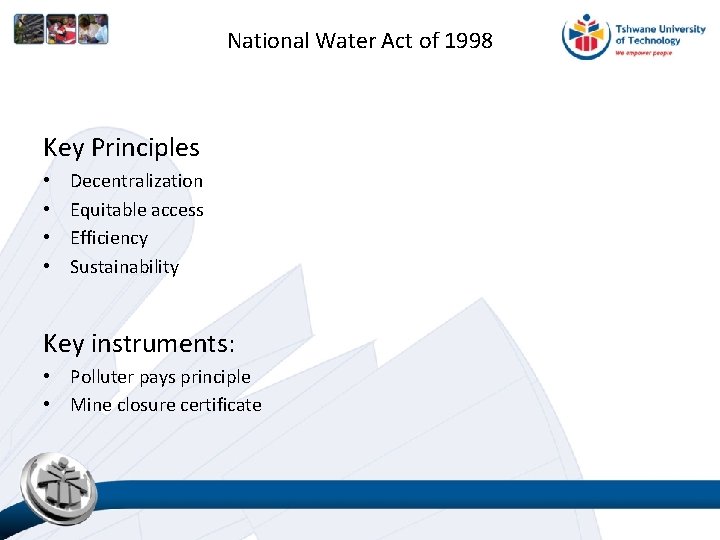
National Water Act of 1998 Key Principles • • Decentralization Equitable access Efficiency Sustainability Key instruments: • Polluter pays principle • Mine closure certificate
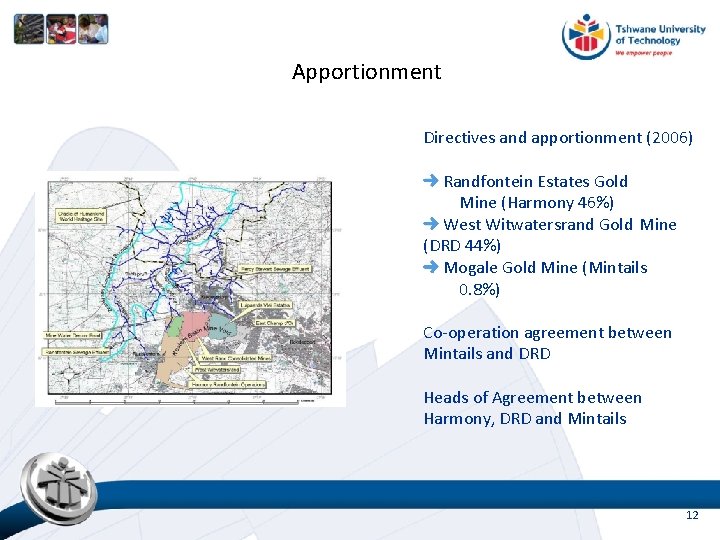
Decant started March 2002 – Apportionment Harmony emergency measures Directives and apportionment (2006) Randfontein Estates Gold Mine (Harmony 46%) West Witwatersrand Gold Mine (DRD 44%) Mogale Gold Mine (Mintails 0. 8%) Co-operation agreement between Mintails and DRD Heads of Agreement between Harmony, DRD and Mintails 12

What is mine water? What is mine water not?
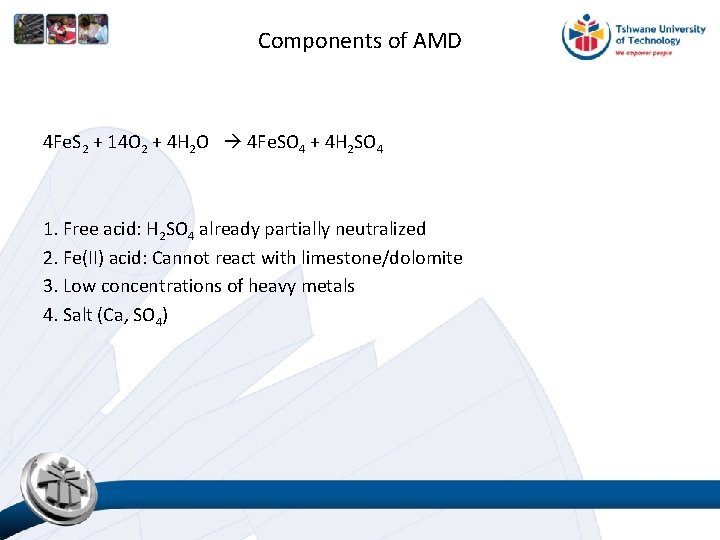
Components of AMD 4 Fe. S 2 + 14 O 2 + 4 H 2 O 4 Fe. SO 4 + 4 H 2 SO 4 1. Free acid: H 2 SO 4 already partially neutralized 2. Fe(II) acid: Cannot react with limestone/dolomite 3. Low concentrations of heavy metals 4. Salt (Ca, SO 4)
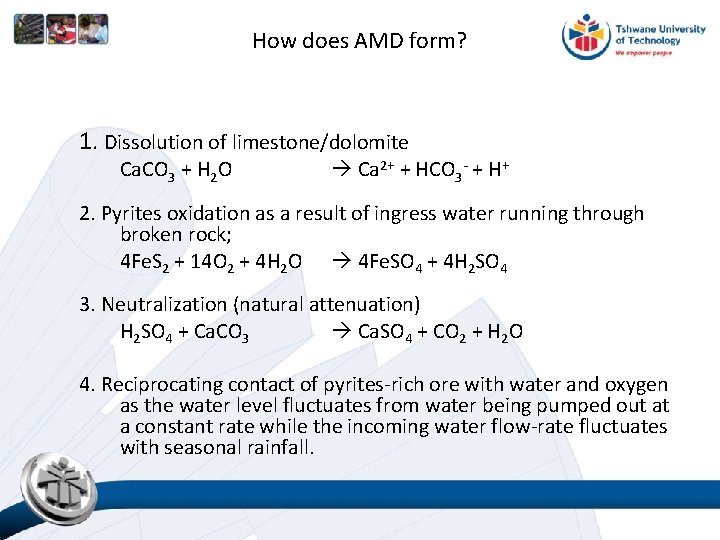
How does AMD form? 1. Dissolution of limestone/dolomite Ca. CO 3 + H 2 O Ca 2+ + HCO 3 - + H+ 2. Pyrites oxidation as a result of ingress water running through broken rock; 4 Fe. S 2 + 14 O 2 + 4 H 2 O 4 Fe. SO 4 + 4 H 2 SO 4 3. Neutralization (natural attenuation) H 2 SO 4 + Ca. CO 3 Ca. SO 4 + CO 2 + H 2 O 4. Reciprocating contact of pyrites-rich ore with water and oxygen as the water level fluctuates from water being pumped out at a constant rate while the incoming water flow-rate fluctuates with seasonal rainfall.
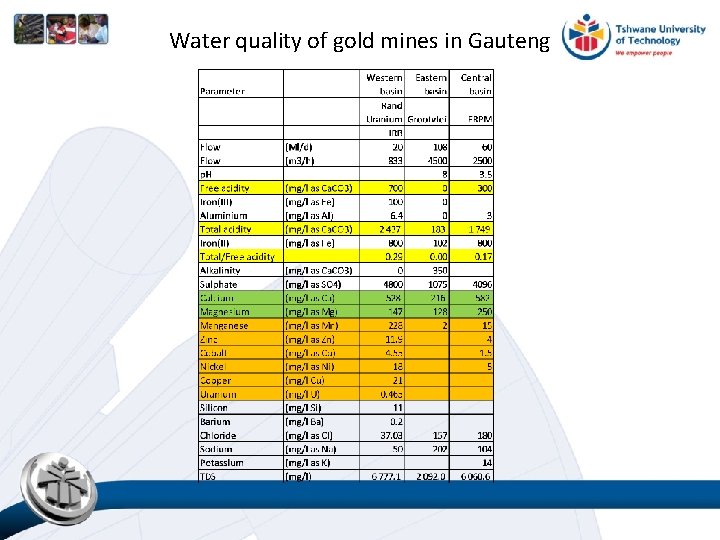
Water quality of gold mines in Gauteng
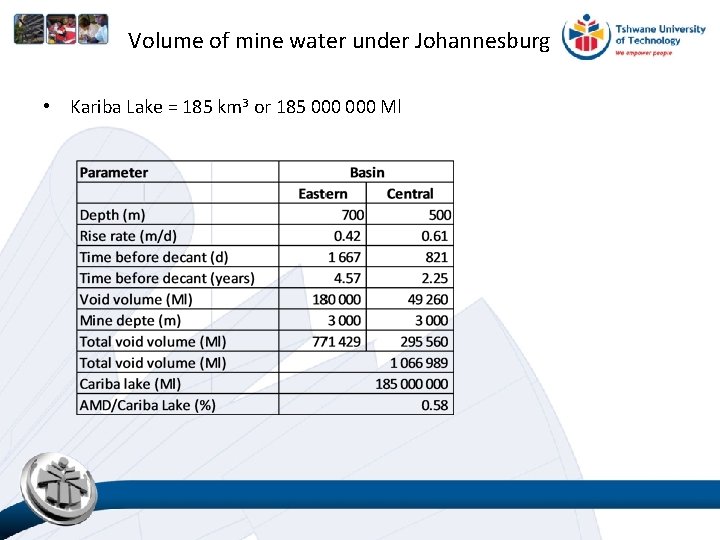
Volume of mine water under Johannesburg • Kariba Lake = 185 km 3 or 185 000 Ml
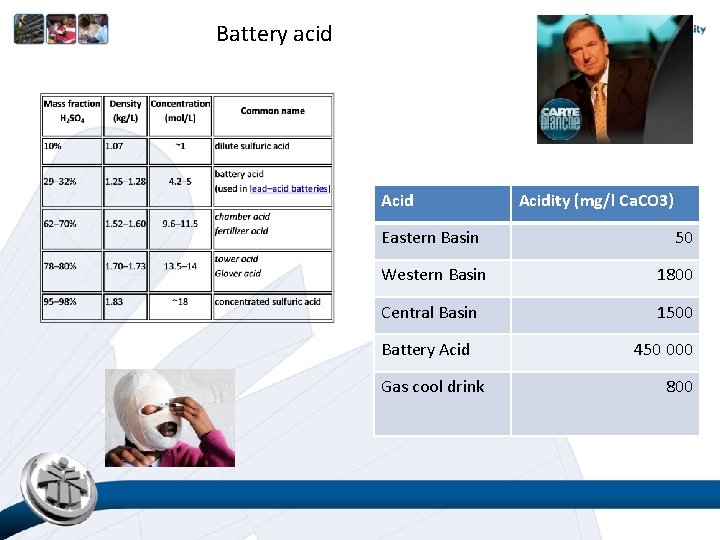
Battery acid Acid Eastern Basin Acidity (mg/l Ca. CO 3) 50 Western Basin 1800 Central Basin 1500 Battery Acid Gas cool drink 450 000 800

Game Reserve put at risk

Four point plan 1. Encourage mining activities to stimulate job creation (reduced labour cost, beneficiation of raw materials). The pumping and treatment cost should be offset by the value of the mined minerals, the treated water, and dissolved by-products reclaimed from the mine water. 2. Immediate implementation of: – limestone neutralization for removal of free acid, iron(II) and partial desalination. – lime treatment for removal of toxic heavy metals and radioactivity, – In the case of Grootvlei Mine, water is already neutralized due to natural attenuation - Passive treatment 3. Implement desalination to meet water demand by 2014. Selected technology based on capital and running costs, performance, process stability. 4. Pumping or not
- Slides: 20