Acid Halides from Carboxylic Acids 1 Synthesis of
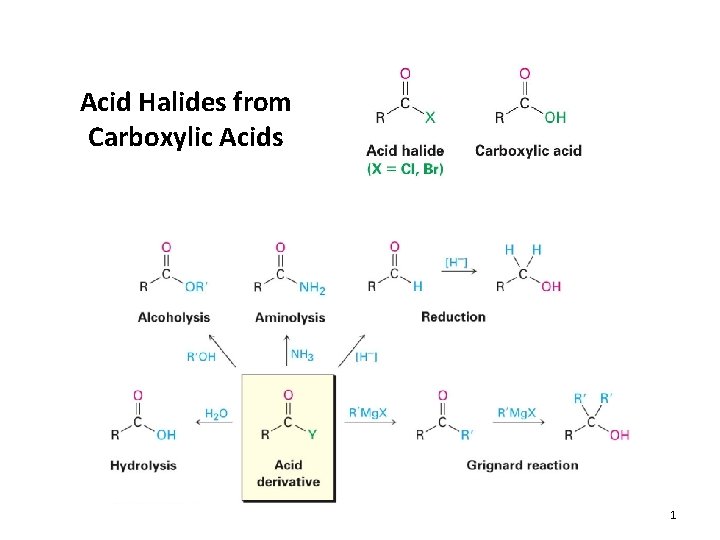
Acid Halides from Carboxylic Acids 1
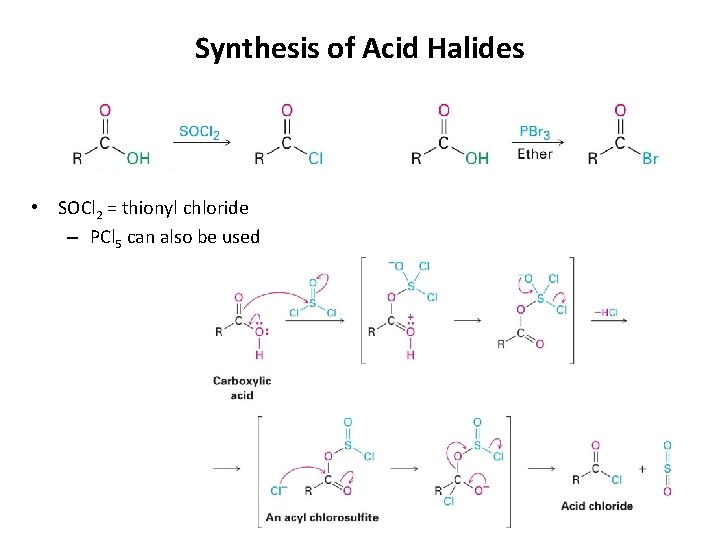
Synthesis of Acid Halides • SOCl 2 = thionyl chloride – PCl 5 can also be used
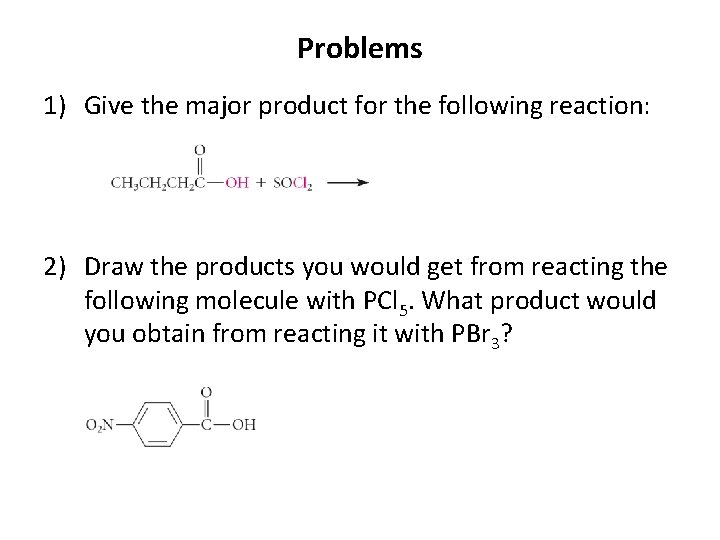
Problems 1) Give the major product for the following reaction: 2) Draw the products you would get from reacting the following molecule with PCl 5. What product would you obtain from reacting it with PBr 3?
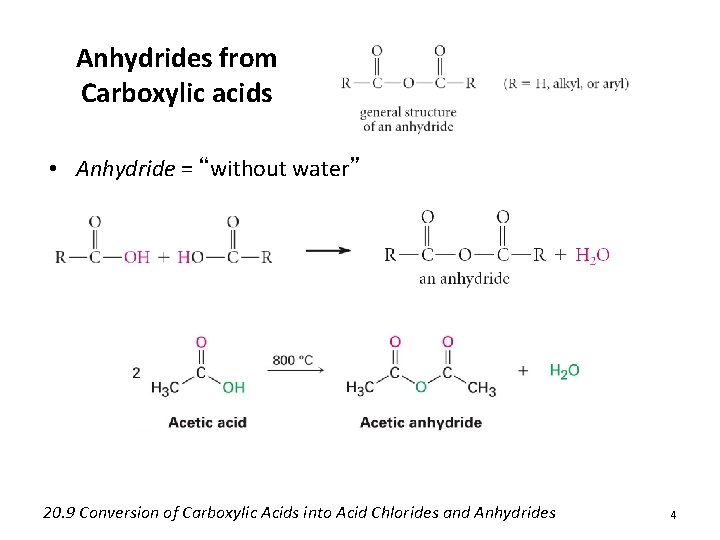
Anhydrides from Carboxylic acids • Anhydride = “without water” 20. 9 Conversion of Carboxylic Acids into Acid Chlorides and Anhydrides 4
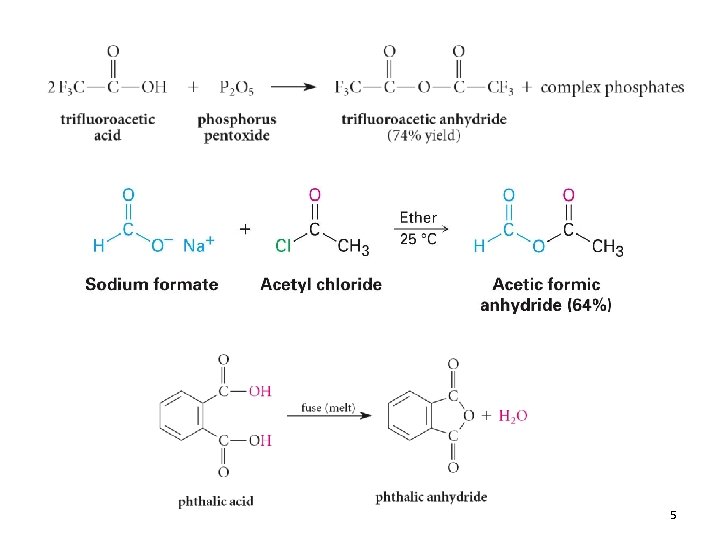
5
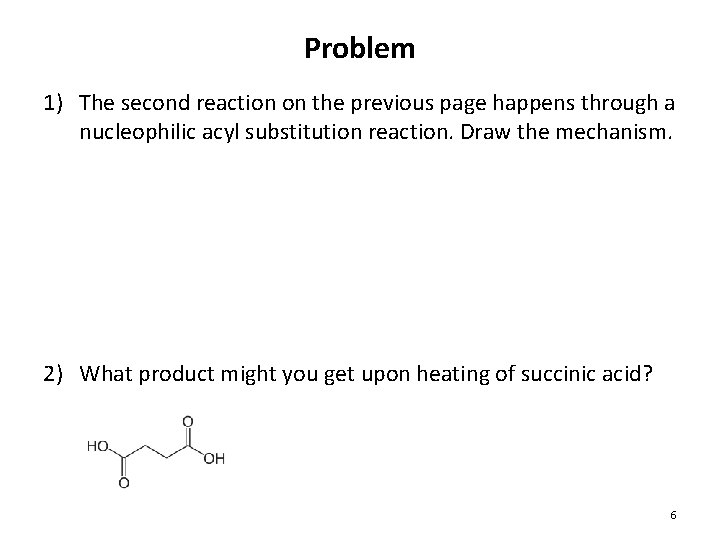
Problem 1) The second reaction on the previous page happens through a nucleophilic acyl substitution reaction. Draw the mechanism. 2) What product might you get upon heating of succinic acid? 6
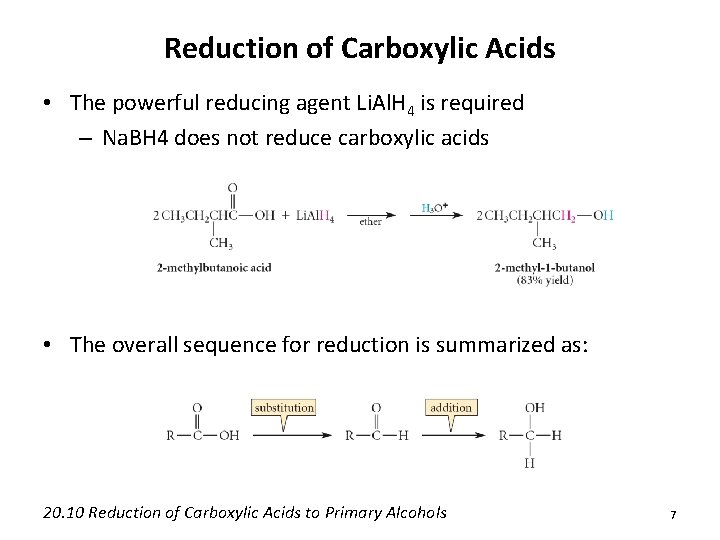
Reduction of Carboxylic Acids • The powerful reducing agent Li. Al. H 4 is required – Na. BH 4 does not reduce carboxylic acids • The overall sequence for reduction is summarized as: 20. 10 Reduction of Carboxylic Acids to Primary Alcohols 7
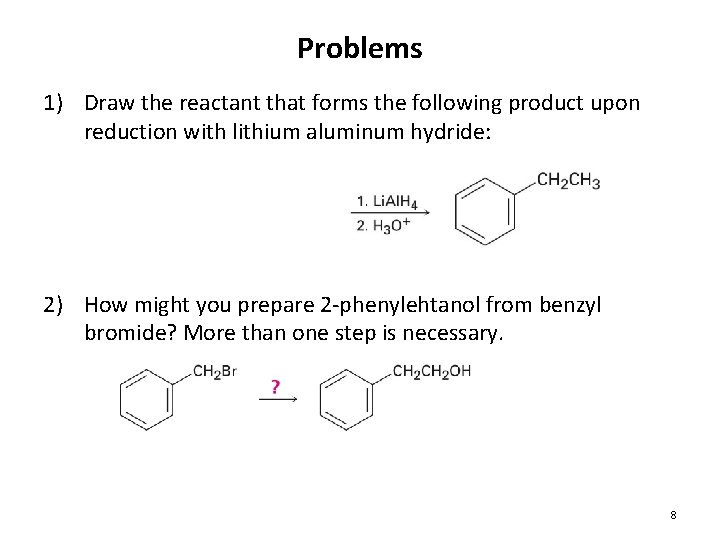
Problems 1) Draw the reactant that forms the following product upon reduction with lithium aluminum hydride: 2) How might you prepare 2 -phenylehtanol from benzyl bromide? More than one step is necessary. 8
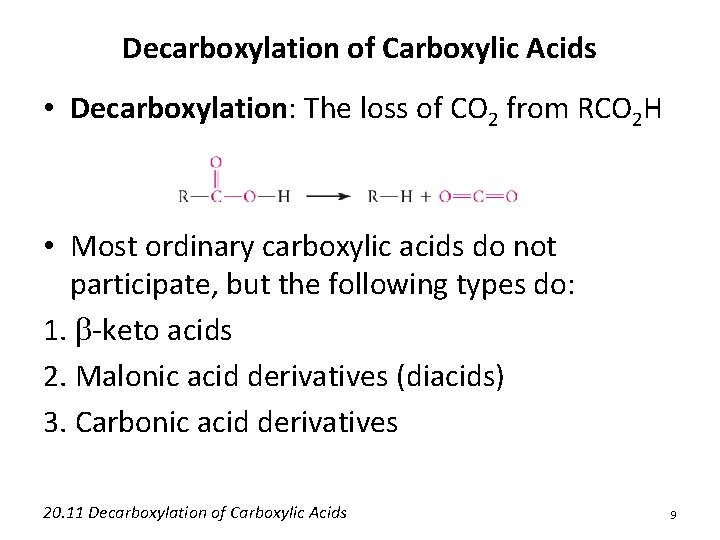
Decarboxylation of Carboxylic Acids • Decarboxylation: The loss of CO 2 from RCO 2 H • Most ordinary carboxylic acids do not participate, but the following types do: 1. b-keto acids 2. Malonic acid derivatives (diacids) 3. Carbonic acid derivatives 20. 11 Decarboxylation of Carboxylic Acids 9
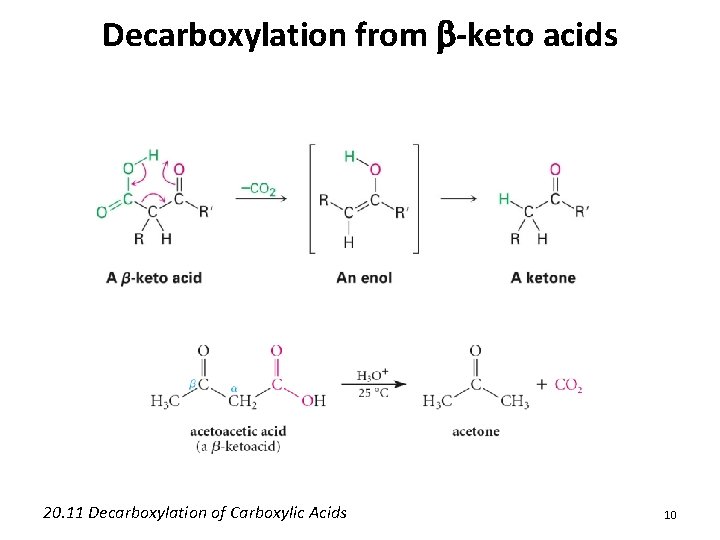
Decarboxylation from b-keto acids 20. 11 Decarboxylation of Carboxylic Acids 10
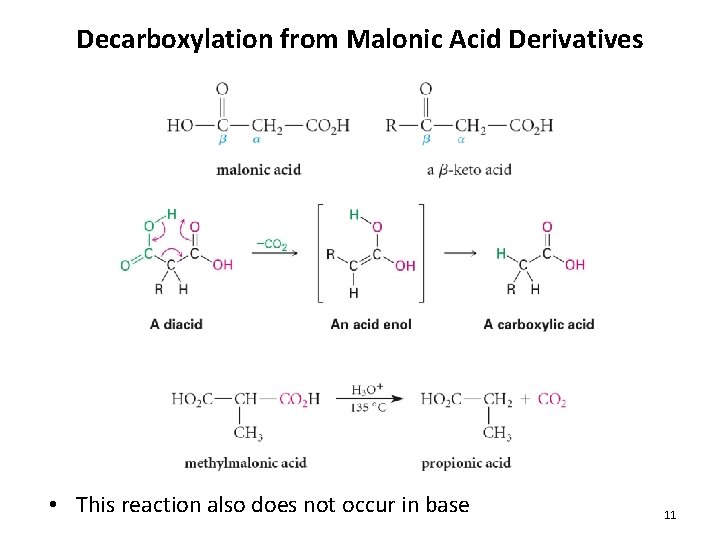
Decarboxylation from Malonic Acid Derivatives • This reaction also does not occur in base 11
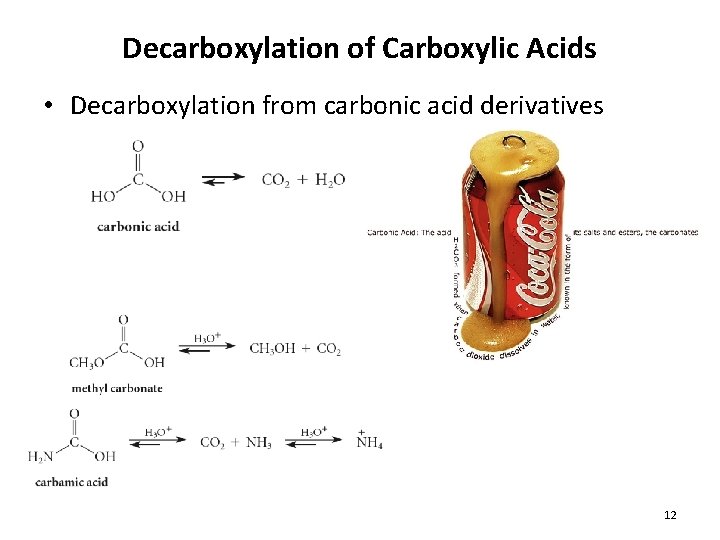
Decarboxylation of Carboxylic Acids • Decarboxylation from carbonic acid derivatives 12
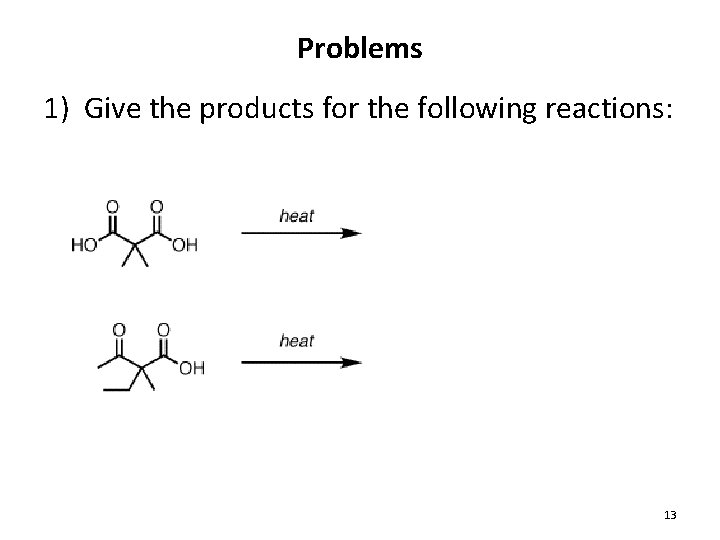
Problems 1) Give the products for the following reactions: 13
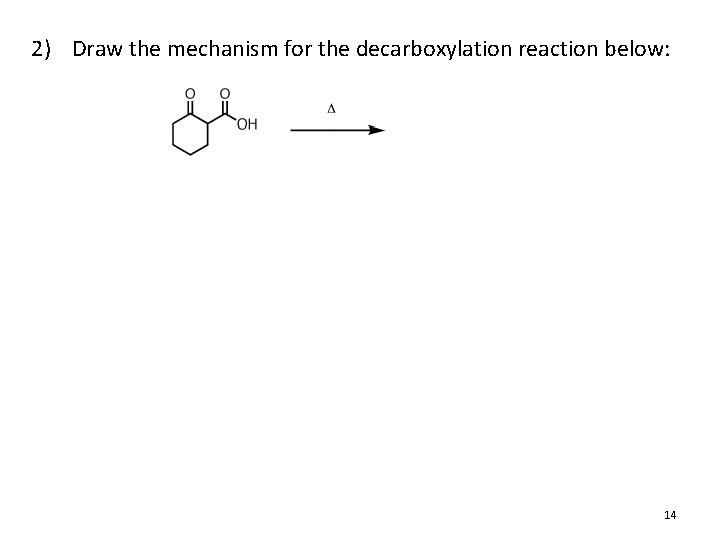
2) Draw the mechanism for the decarboxylation reaction below: 14
- Slides: 14