Acid Definitions Lewis Acid BrnstedLowry Arrhenius acids Arrhenius
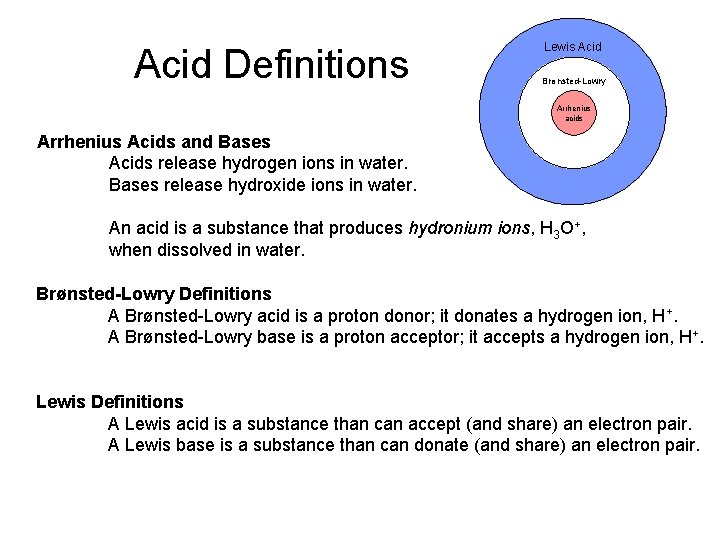
Acid Definitions Lewis Acid Brønsted-Lowry Arrhenius acids Arrhenius Acids and Bases Acids release hydrogen ions in water. Bases release hydroxide ions in water. An acid is a substance that produces hydronium ions, H 3 O+, when dissolved in water. Brønsted-Lowry Definitions A Brønsted-Lowry acid is a proton donor; it donates a hydrogen ion, H+. A Brønsted-Lowry base is a proton acceptor; it accepts a hydrogen ion, H+. Lewis Definitions A Lewis acid is a substance than can accept (and share) an electron pair. A Lewis base is a substance than can donate (and share) an electron pair.
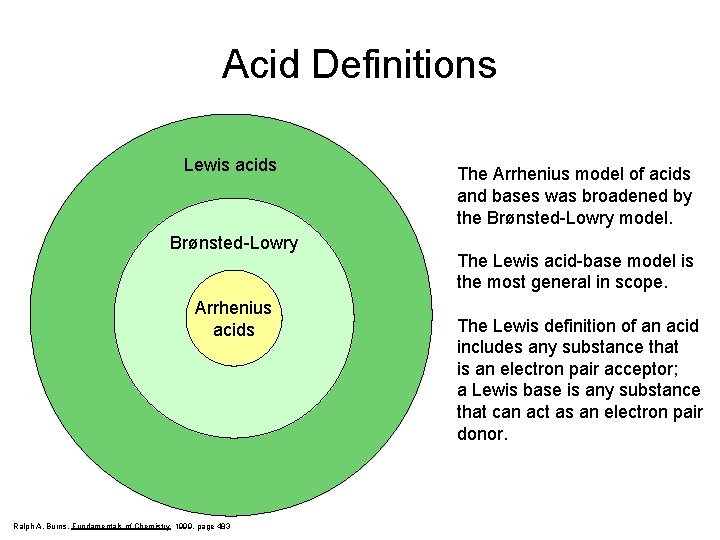
Acid Definitions Lewis acids Brønsted-Lowry Arrhenius acids Ralph A. Burns, Fundamentals of Chemistry 1999, page 483 The Arrhenius model of acids and bases was broadened by the Brønsted-Lowry model. The Lewis acid-base model is the most general in scope. The Lewis definition of an acid includes any substance that is an electron pair acceptor; a Lewis base is any substance that can act as an electron pair donor.
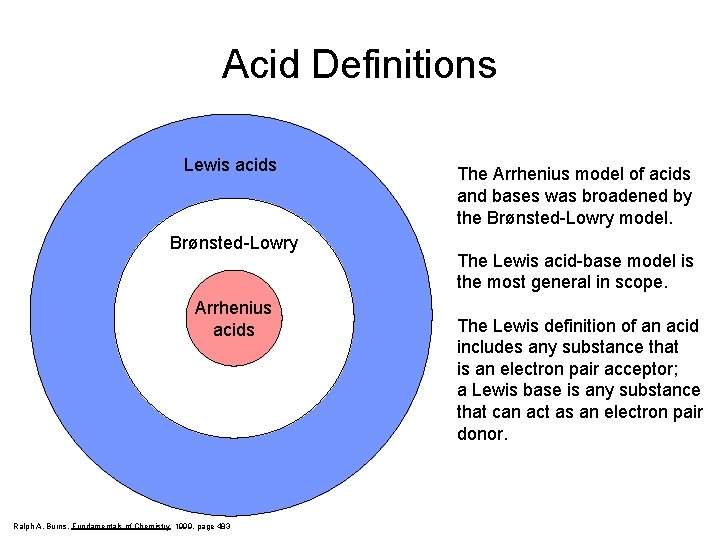
Acid Definitions Lewis acids Brønsted-Lowry Arrhenius acids Ralph A. Burns, Fundamentals of Chemistry 1999, page 483 The Arrhenius model of acids and bases was broadened by the Brønsted-Lowry model. The Lewis acid-base model is the most general in scope. The Lewis definition of an acid includes any substance that is an electron pair acceptor; a Lewis base is any substance that can act as an electron pair donor.
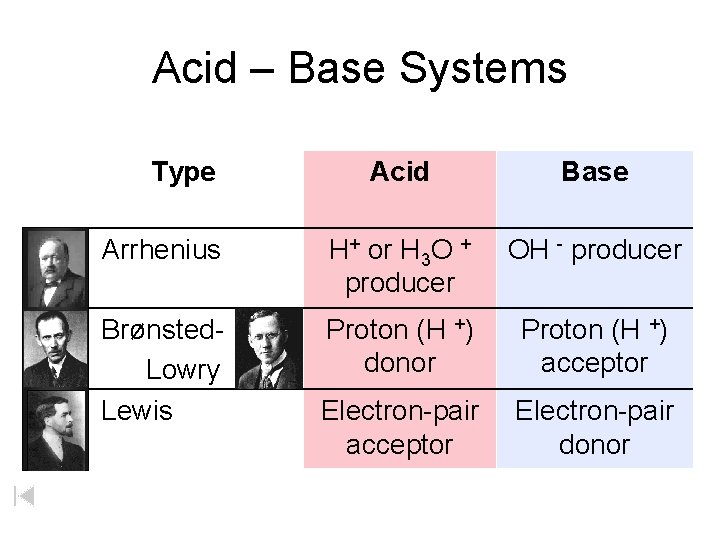
Acid – Base Systems Type Acid Base Arrhenius H+ or H 3 O + producer OH - producer Brønsted. Lowry Lewis Proton (H +) donor Proton (H +) acceptor Electron-pair donor
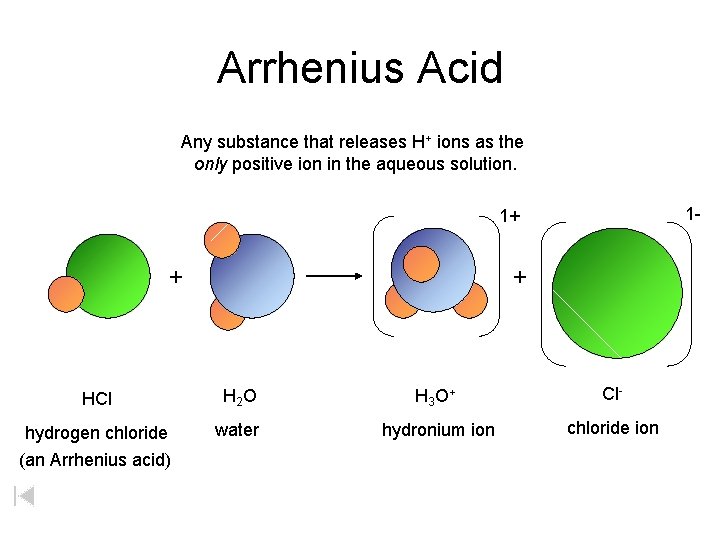
Arrhenius Acid Any substance that releases H+ ions as the only positive ion in the aqueous solution. 1 - 1+ + + HCl H 2 O H 3 O + Cl- hydrogen chloride (an Arrhenius acid) water hydronium ion chloride ion
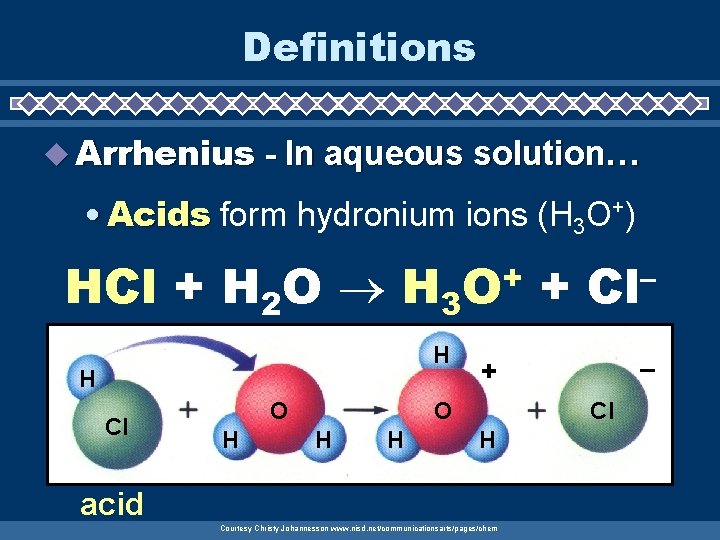
Definitions u Arrhenius - In aqueous solution… • Acids form hydronium ions (H 3 O+) HCl + H 2 O H 3 O+ + Cl– H H Cl O H H – + Cl H acid Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem
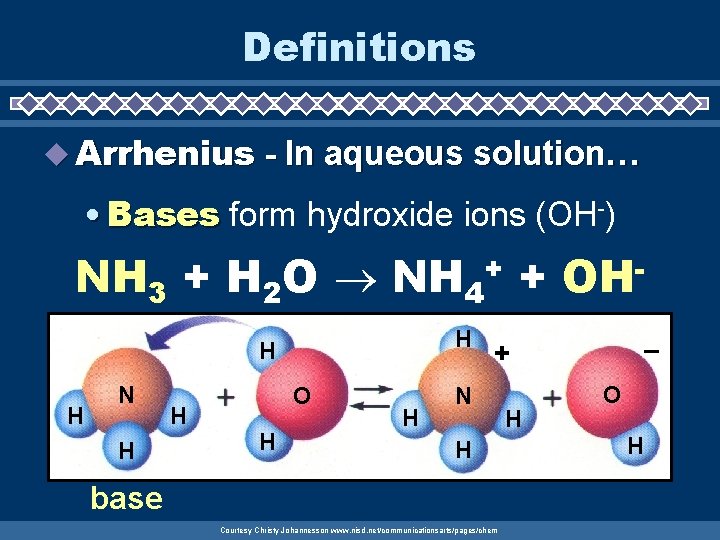
Definitions u Arrhenius - In aqueous solution… • Bases form hydroxide ions (OH-) NH 3 + H 2 O NH 4 + + H H H N H H OH – + N H base Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem H O H
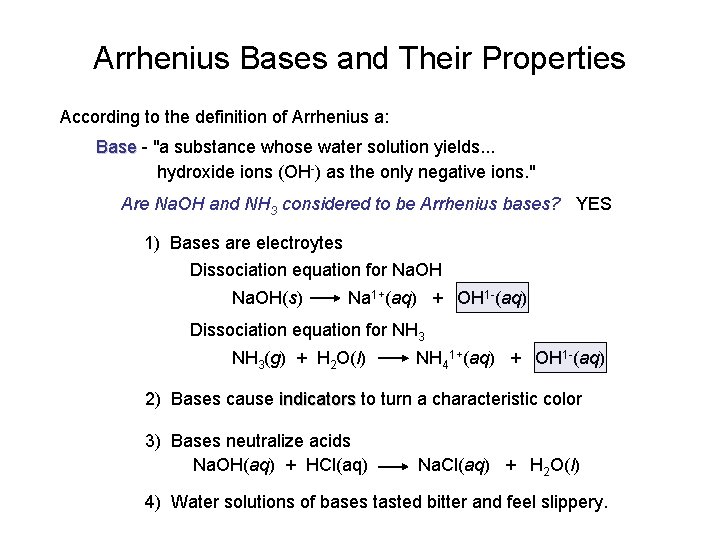
Arrhenius Bases and Their Properties According to the definition of Arrhenius a: Base - "a substance whose water solution yields. . . hydroxide ions (OH-) as the only negative ions. " Are Na. OH and NH 3 considered to be Arrhenius bases? YES 1) Bases are electroytes Dissociation equation for Na. OH(s) Na 1+(aq) + OH 1 -(aq) Dissociation equation for NH 3(g) + H 2 O(l) NH 41+(aq) + OH 1 -(aq) 2) Bases cause indicators to turn a characteristic color 3) Bases neutralize acids Na. OH(aq) + HCl(aq) Na. Cl(aq) + H 2 O(l) 4) Water solutions of bases tasted bitter and feel slippery.
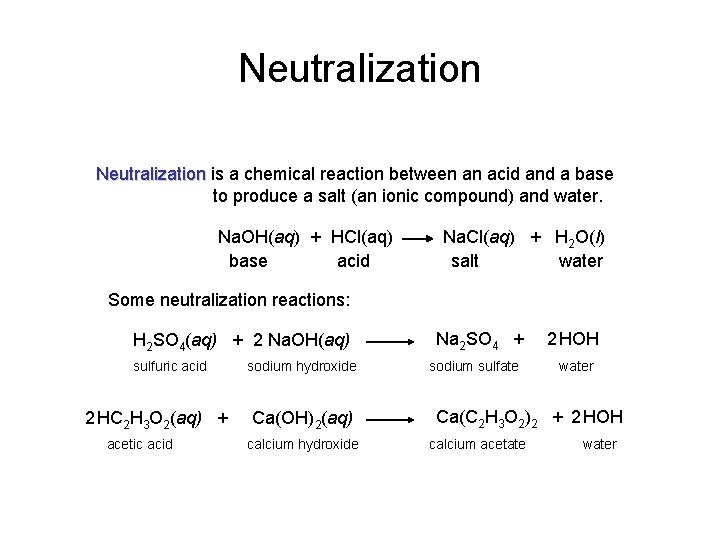
Neutralization is a chemical reaction between an acid and a base to produce a salt (an ionic compound) and water. Na. OH(aq) + HCl(aq) base acid Na. Cl(aq) + H 2 O(l) salt water Some neutralization reactions: H 2 SO 4(aq) + 2 Na. OH(aq) sulfuric acid 2 HC 2 H 3 O 2(aq) + acetic acid sodium hydroxide Ca(OH)2(aq) calcium hydroxide Na 2 SO 4 + sodium sulfate 2 HOH water Ca(C 2 H 3 O 2)2 + 2 HOH calcium acetate water
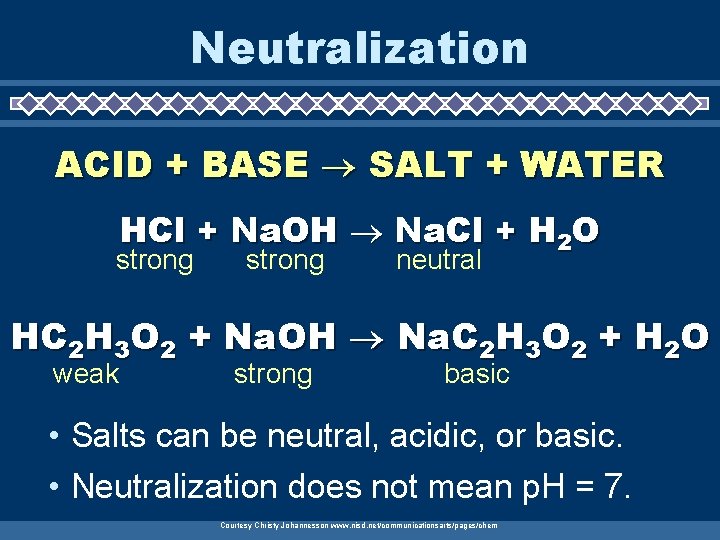
Neutralization ACID + BASE SALT + WATER HCl + Na. OH Na. Cl + H 2 O strong neutral HC 2 H 3 O 2 + Na. OH Na. C 2 H 3 O 2 + H 2 O weak strong basic • Salts can be neutral, acidic, or basic. • Neutralization does not mean p. H = 7. Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem
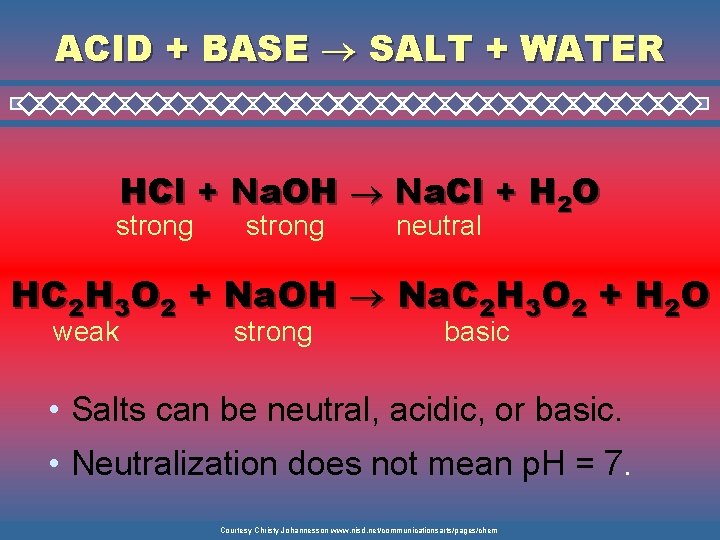
ACID + BASE SALT + WATER HCl + Na. OH Na. Cl + H 2 O strong neutral HC 2 H 3 O 2 + Na. OH Na. C 2 H 3 O 2 + H 2 O weak strong basic • Salts can be neutral, acidic, or basic. • Neutralization does not mean p. H = 7. Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem
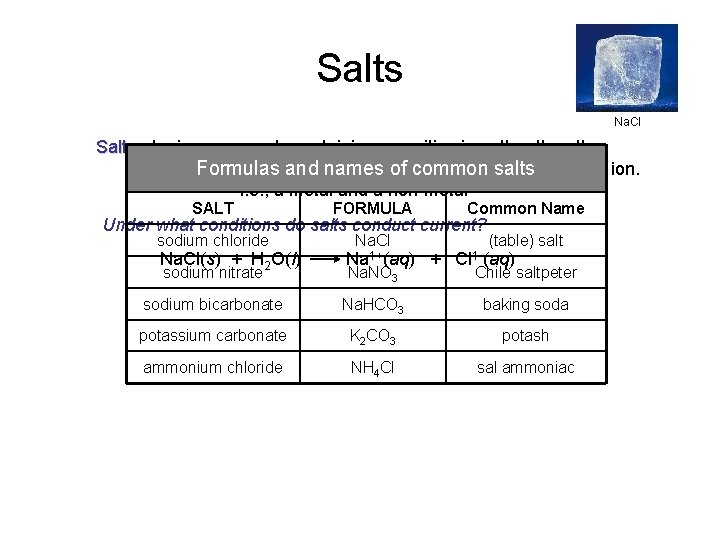
Salts Na. Cl Salts - Ionic compounds containing a positive ion other than the hydrogen ion andand a negative than salts the hydroxide ion. Formulas namesion of other common i. e. , a metal and a non-metal SALT FORMULA sodium chloride Na. Cl Common Name Under what conditions do salts conduct current? Na. Cl(s) + H 2 O(l) sodium nitrate Na 1+(aq) Na. NO 3 + (table) salt Cl 1 -(aq) Chile saltpeter sodium bicarbonate Na. HCO 3 baking soda potassium carbonate K 2 CO 3 potash ammonium chloride NH 4 Cl sal ammoniac
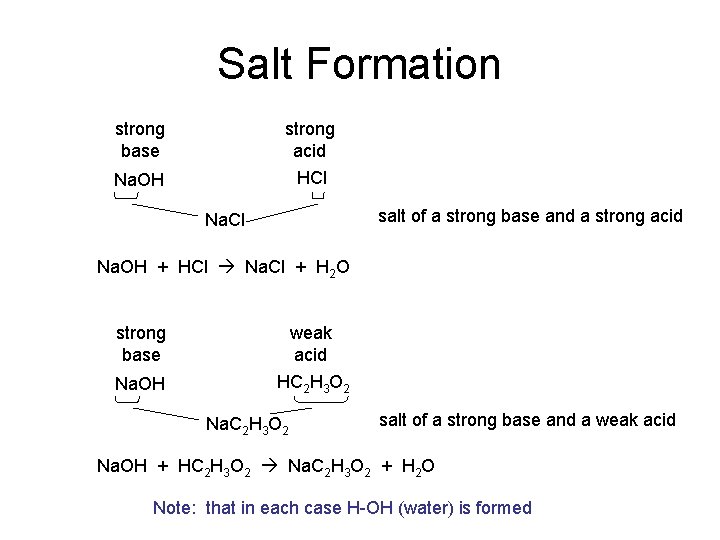
Salt Formation strong base strong acid Na. OH HCl salt of a strong base and a strong acid Na. Cl Na. OH + HCl Na. Cl + H 2 O strong base weak acid Na. OH HC 2 H 3 O 2 Na. C 2 H 3 O 2 salt of a strong base and a weak acid Na. OH + HC 2 H 3 O 2 Na. C 2 H 3 O 2 + H 2 O Note: that in each case H-OH (water) is formed
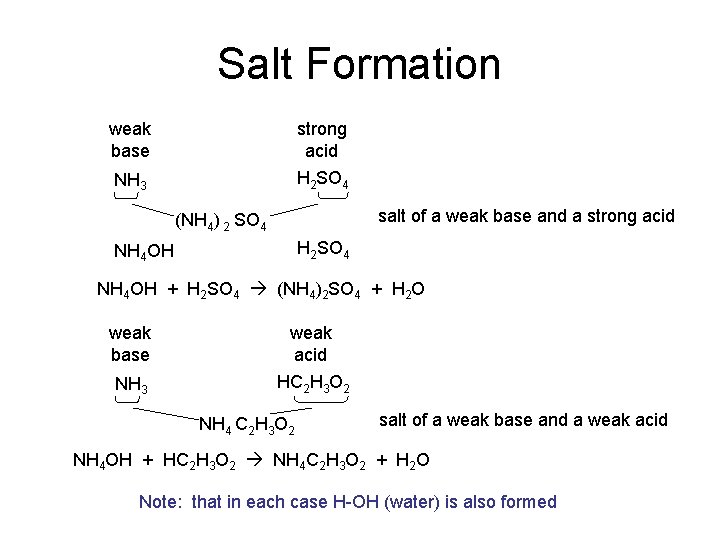
Salt Formation weak base strong acid NH 3 H 2 SO 4 salt of a weak base and a strong acid (NH 4) 2 SO 4 H 2 SO 4 NH 4 OH + H 2 SO 4 (NH 4)2 SO 4 + H 2 O weak base weak acid NH 3 HC 2 H 3 O 2 NH 4 C 2 H 3 O 2 salt of a weak base and a weak acid NH 4 OH + HC 2 H 3 O 2 NH 4 C 2 H 3 O 2 + H 2 O Note: that in each case H-OH (water) is also formed
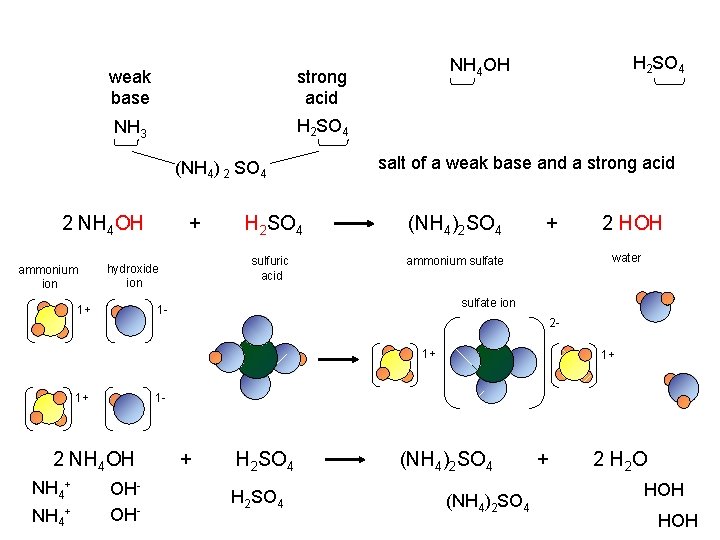
weak base strong acid NH 3 H 2 SO 4 (NH 4) 2 SO 4 2 NH 4 OH ammonium ion + H 2 SO 4 sulfuric acid hydroxide ion salt of a weak base and a strong acid (NH 4)2 SO 4 + sulfate ion 21+ 2 NH 4 OH NH 4+ 1+ 1 - 1+ NH 4+ 2 HOH water ammonium sulfate 1 - 1+ H 2 SO 4 NH 4 OH OHOH- + H 2 SO 4 (NH 4)2 SO 4 + 2 H 2 O HOH
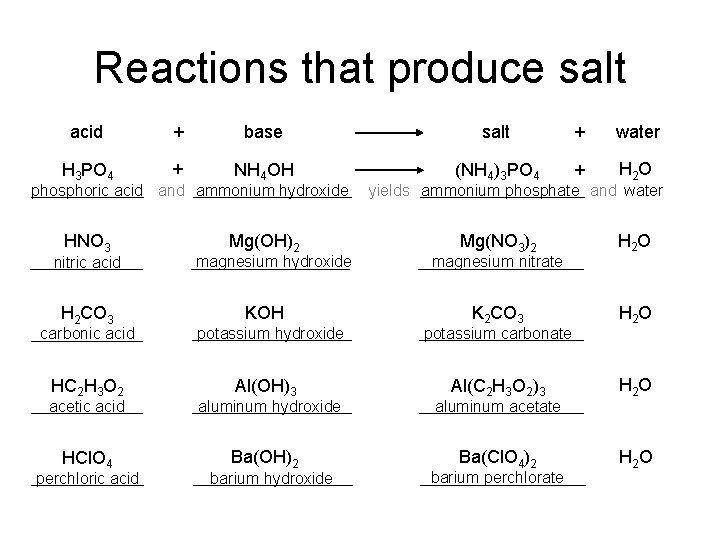
Reactions that produce salt acid + base salt + water H 3 PO 4 + NH 4 OH (NH 4)3 PO 4 + H 2 O phosphoric acid and ammonium hydroxide HNO 3 nitric acid H 2 CO 3 carbonic acid HC 2 H 3 O 2 acetic acid HCl. O 4 perchloric acid Mg(OH)2 magnesium hydroxide KOH potassium hydroxide Al(OH)3 aluminum hydroxide Ba(OH)2 barium hydroxide yields ammonium phosphate and water Mg(NO 3)2 H 2 O K 2 CO 3 H 2 O Al(C 2 H 3 O 2)3 H 2 O Ba(Cl. O 4)2 H 2 O magnesium nitrate potassium carbonate aluminum acetate barium perchlorate
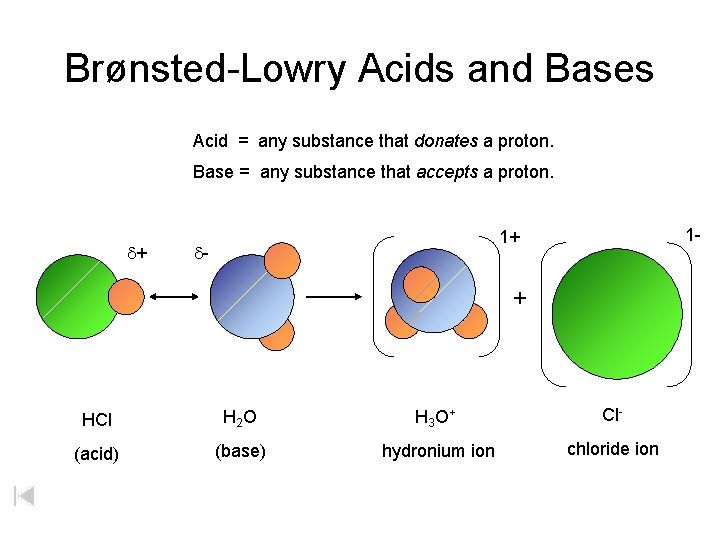
Brønsted-Lowry Acids and Bases Acid = any substance that donates a proton. Base = any substance that accepts a proton. d+ 1 - 1+ d- + HCl H 2 O H 3 O + Cl- (acid) (base) hydronium ion chloride ion
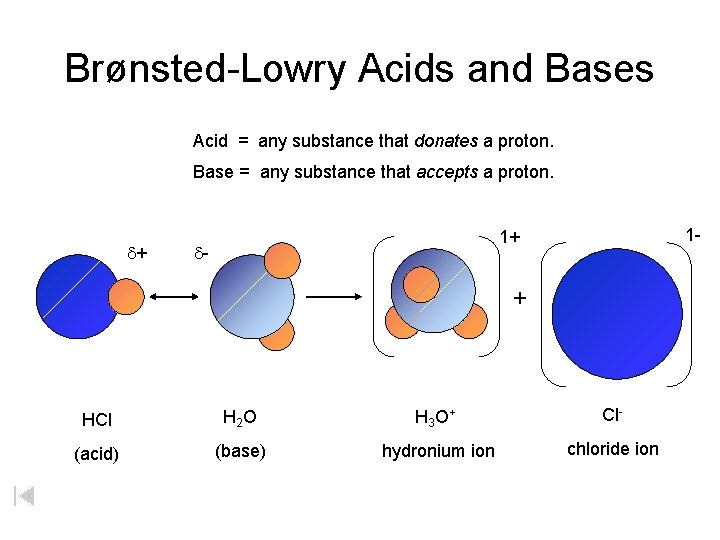
Brønsted-Lowry Acids and Bases Acid = any substance that donates a proton. Base = any substance that accepts a proton. d+ 1 - 1+ d- + HCl H 2 O H 3 O + Cl- (acid) (base) hydronium ion chloride ion
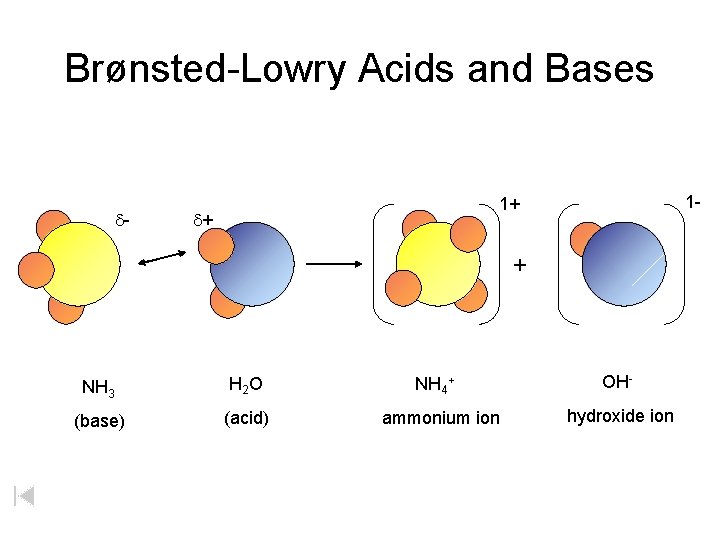
Brønsted-Lowry Acids and Bases d- 1 - 1+ d+ + NH 3 H 2 O (base) (acid) NH 4+ ammonium ion OHhydroxide ion
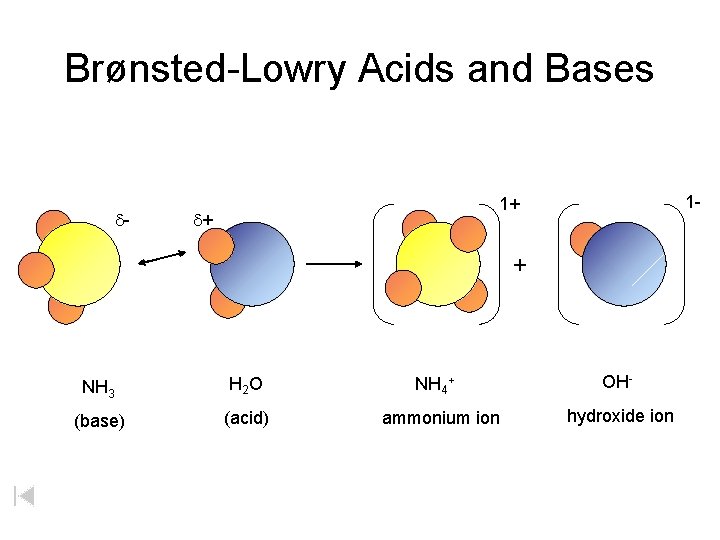
Brønsted-Lowry Acids and Bases d- 1 - 1+ d+ + NH 3 H 2 O (base) (acid) NH 4+ ammonium ion OHhydroxide ion
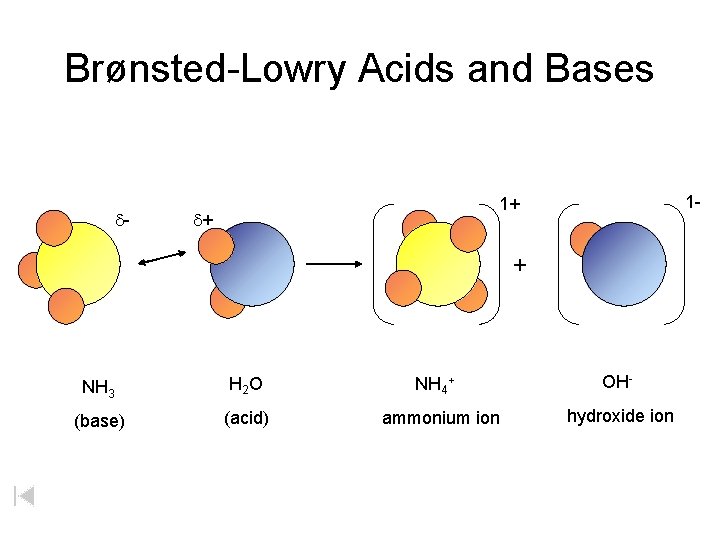
Brønsted-Lowry Acids and Bases d- 1 - 1+ d+ + NH 3 H 2 O (base) (acid) NH 4+ ammonium ion OHhydroxide ion
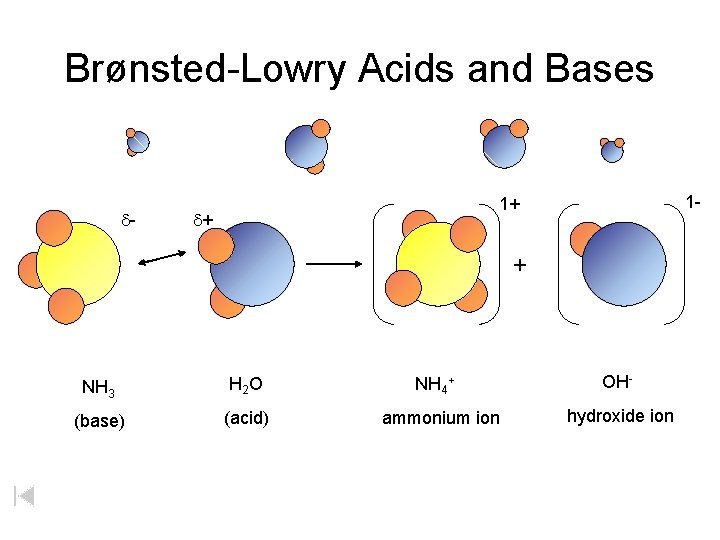
Brønsted-Lowry Acids and Bases d- 1 - 1+ d+ + NH 3 H 2 O (base) (acid) NH 4+ ammonium ion OHhydroxide ion
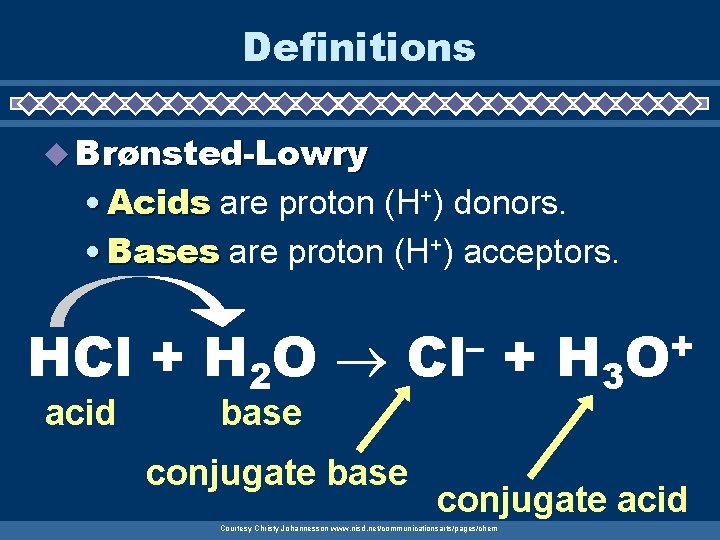
Definitions u Brønsted-Lowry • Acids are proton (H+) donors. • Bases are proton (H+) acceptors. HCl + H 2 O acid – Cl base conjugate base + H 3 + O conjugate acid Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem
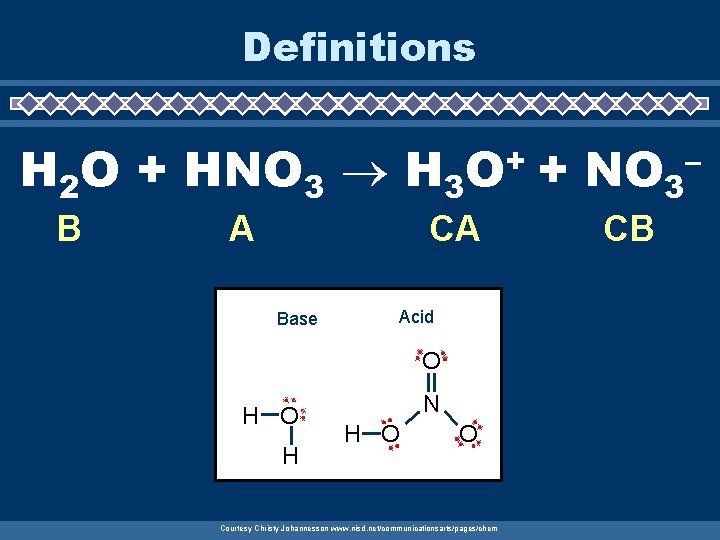
Definitions H 2 O + HNO 3 H 3 B A + O CA Base Acid O H N H O O Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem + NO 3 CB –
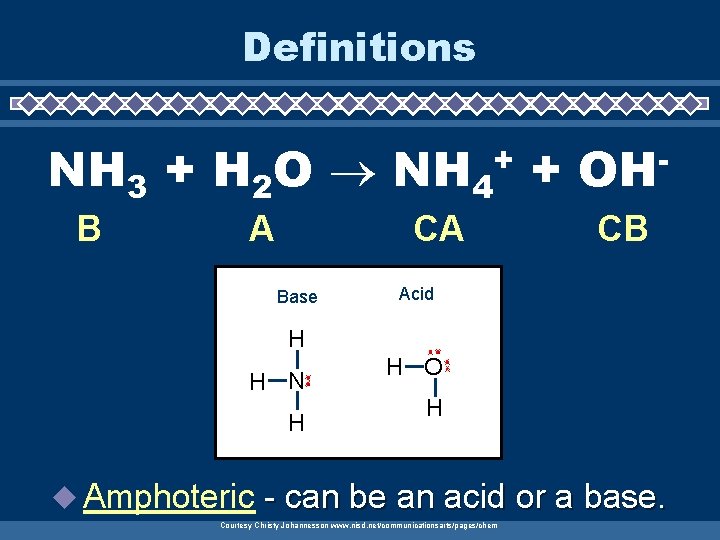
Definitions NH 3 + H 2 O NH 4 + + B A CA Base OH CB Acid H H N H u Amphoteric H O H - can be an acid or a base. Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem
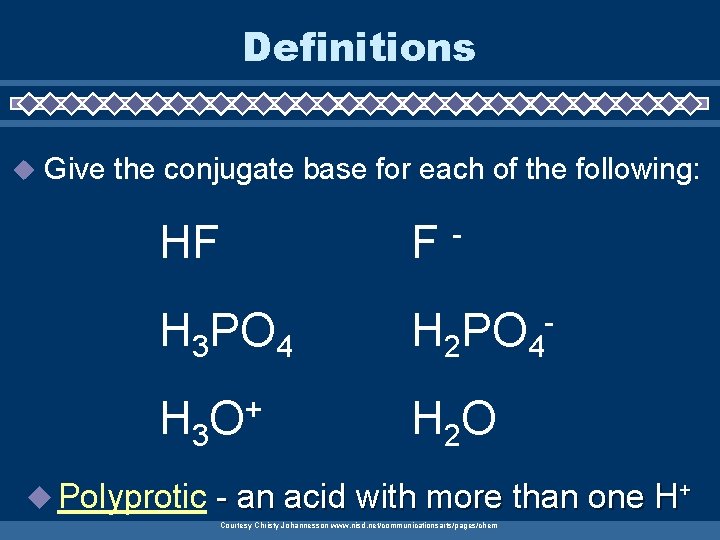
Definitions u Give the conjugate base for each of the following: HF F H 3 PO 4 H 2 PO 4 - H 3 O + H 2 O u Polyprotic - - an acid with more than one H+ Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem
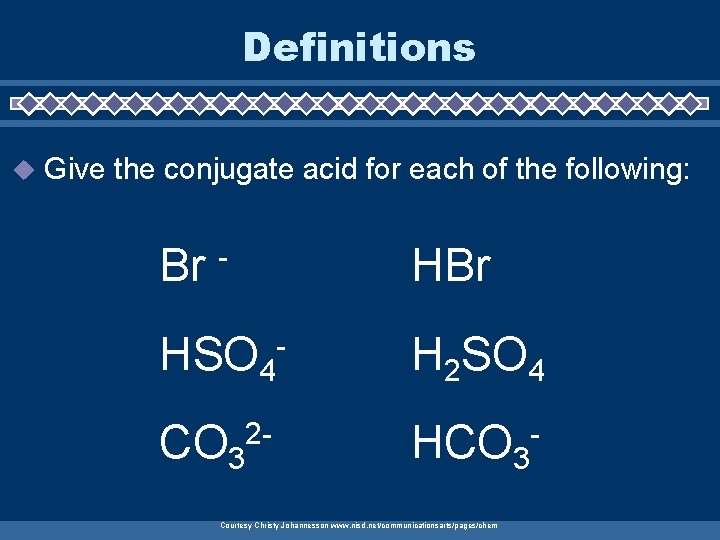
Definitions u Give the conjugate acid for each of the following: Br HBr - HSO 4 CO 32 - - H 2 SO 4 HCO 3 - Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem
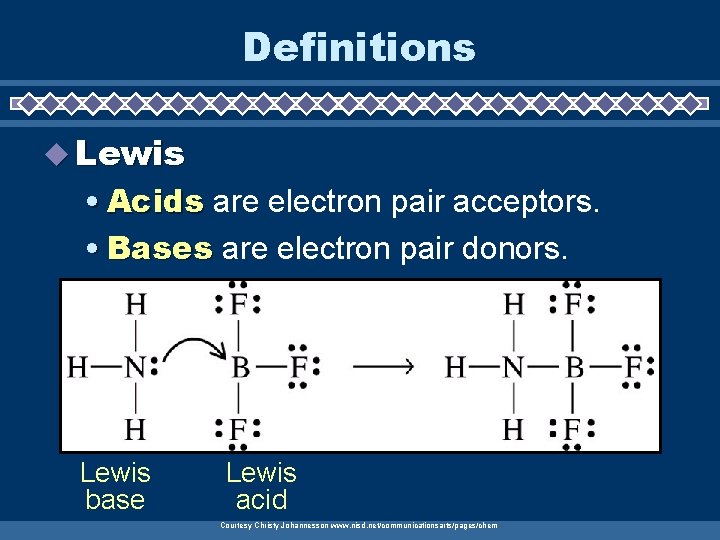
Definitions u Lewis • Acids are electron pair acceptors. • Bases are electron pair donors. Lewis base Lewis acid Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem
- Slides: 28