ACID CHLORIDE Presentation and Speech Piper Nanouski What





- Slides: 8

ACID CHLORIDE Presentation and Speech: Piper Nanouski

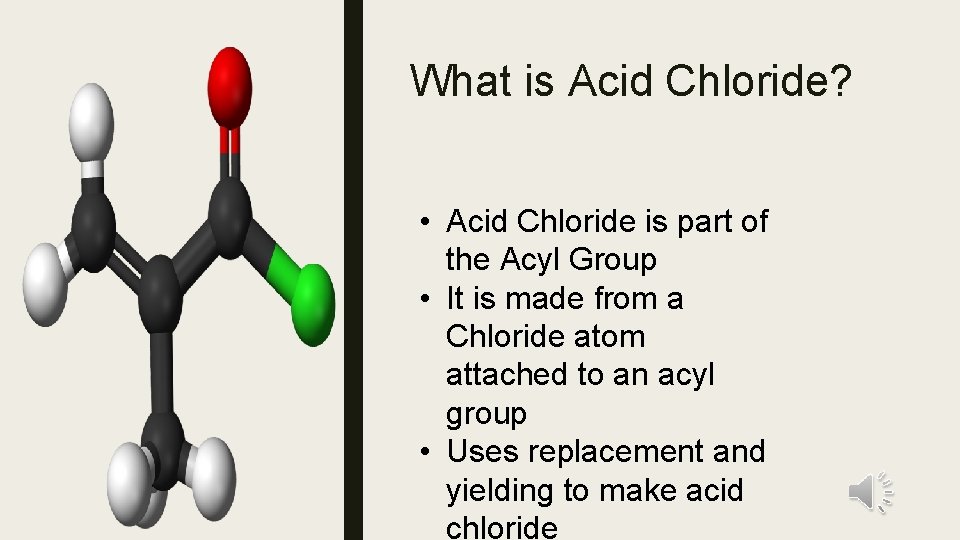
What is Acid Chloride? • Acid Chloride is part of the Acyl Group • It is made from a Chloride atom attached to an acyl group • Uses replacement and yielding to make acid chloride

■ The Acyl group is represented by ROC. ■ ROC is made up of carbon and R being bonded by a single bond. ■ Larger Molecules can be bonded to the oxygen and the carbon ■ Acyl groups are made from when one or more hydroxyl groups are taken out of oxoacid – that is how the acyl group is formed Acyl Group

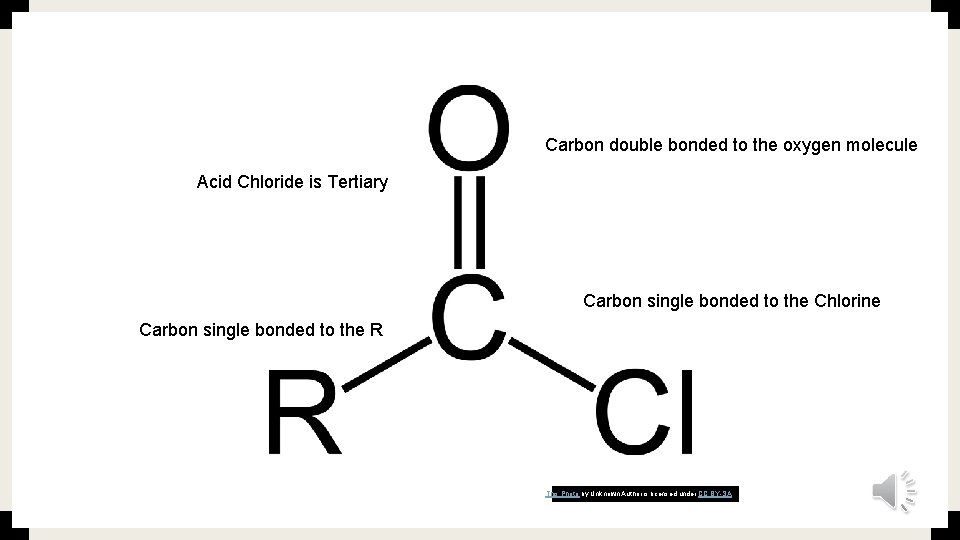
Carbon double bonded to the oxygen molecule Acid Chloride is Tertiary Carbon single bonded to the Chlorine Carbon single bonded to the R This Photo by Unknown Author is licensed under CC BY-SA

The Molecular Formulas ■ Acyl group, which is the functional group is RCO ■ RCO means that R is single bonded to carbon and carbon is double bonded to oxygen ■ For Acid Chloride the Formula is RCOCL ■ Which means the R is single bonded to carbon, the carbon is double bonded to oxygen and the oxygen is single bonded to the chlorine. ■ https: //study. com/academy/lesson/acid-chloride-formation-reaction. html

Compounds to find in the Acyl Group ■ acetyl chloride (CH 3 COCl) ■ benzoyl chloride (C 6 H 5 COCl) ■ Amides (RC(O)NR 2) ■ esters (RC(O)OR′) ■ ketones (RC(O)R) ■ aldehydes (RC(O)H)

Characteristics and Functional Properties of Acyl Group ■ The acyl group is derived from the carboxyl acid ■ R represents an alkyl group that is linked to the carbon atom of the group by a single bond ■ acyl groups are attached to a larger molecular fragment ■ acyl groups can in principle be derived from other types of acids such as sulfonic acids, phosphonic acids ■ It contains a double-bonded oxygen atom and an alkyl group

Unique Characteristics 1. Aversion- creating hydrogen bonds 2. Electronegative- Loves to create bonds with electrons 3. Nucleophilic- Draws electrons into oxygen