Acid Base Titrations Lesson 4 In a titration

Acid Base Titrations Lesson 4

In a titration the molarity of one chemical is determined by reacting it with another one with known molarity (standard).
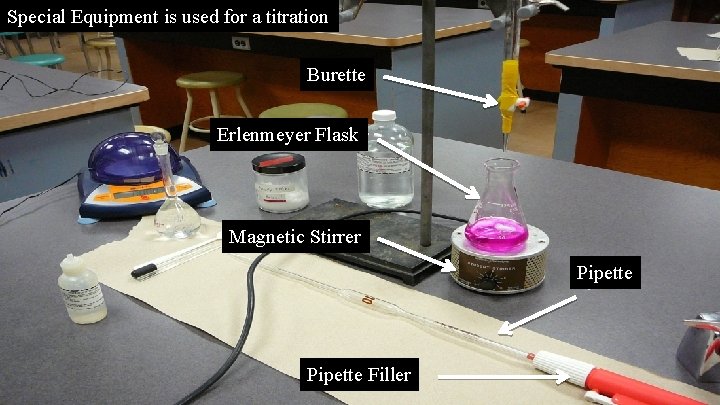
Special Equipment is used for a titration Burette Erlenmeyer Flask Magnetic Stirrer Pipette Filler

Digital Balance Solid acid to make a standard solution Volumetric Flask
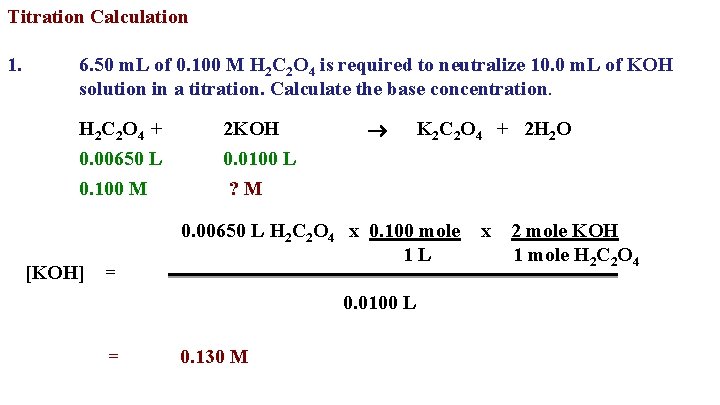
Titration Calculation 1. 6. 50 m. L of 0. 100 M H 2 C 2 O 4 is required to neutralize 10. 0 m. L of KOH solution in a titration. Calculate the base concentration. H 2 C 2 O 4 + 2 KOH 0. 00650 L 0. 100 M 0. 0100 L ? M [KOH] = 0. 00650 L H 2 C 2 O 4 x 0. 100 mole 1 L 0. 0100 L = K 2 C 2 O 4 + 2 H 2 O 0. 130 M x 2 mole KOH 1 mole H 2 C 2 O 4
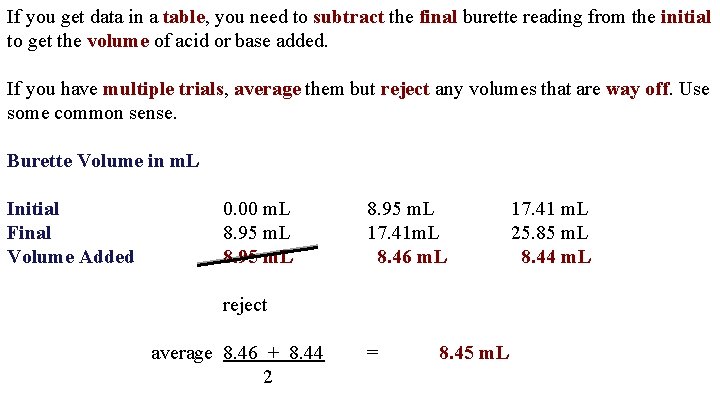
If you get data in a table, you need to subtract the final burette reading from the initial to get the volume of acid or base added. If you have multiple trials, average them but reject any volumes that are way off. Use some common sense. Burette Volume in m. L Initial Final Volume Added 0. 00 m. L 8. 95 m. L 17. 41 m. L 8. 46 m. L reject average 8. 46 + 8. 44 2 = 8. 45 m. L 17. 41 m. L 25. 85 m. L 8. 44 m. L

Now it's your turn to try one!
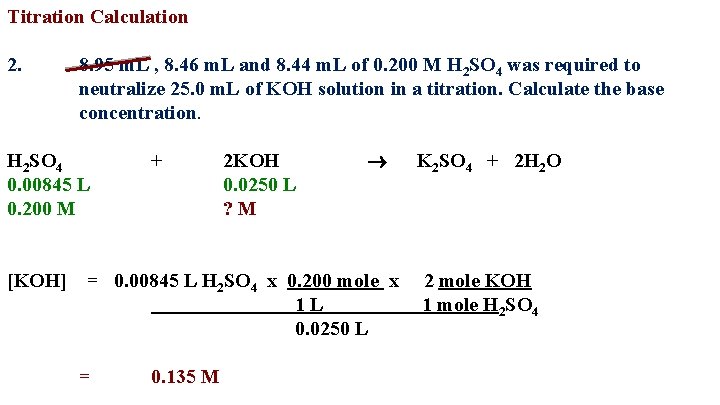
Titration Calculation 2. 8. 95 m. L , 8. 46 m. L and 8. 44 m. L of 0. 200 M H 2 SO 4 was required to neutralize 25. 0 m. L of KOH solution in a titration. Calculate the base concentration. H 2 SO 4 0. 00845 L 0. 200 M [KOH] + 2 KOH 0. 0250 L ? M = 0. 00845 L H 2 SO 4 x 0. 200 mole x 1 L 0. 0250 L = 0. 135 M K 2 SO 4 + 2 H 2 O 2 mole KOH 1 mole H 2 SO 4
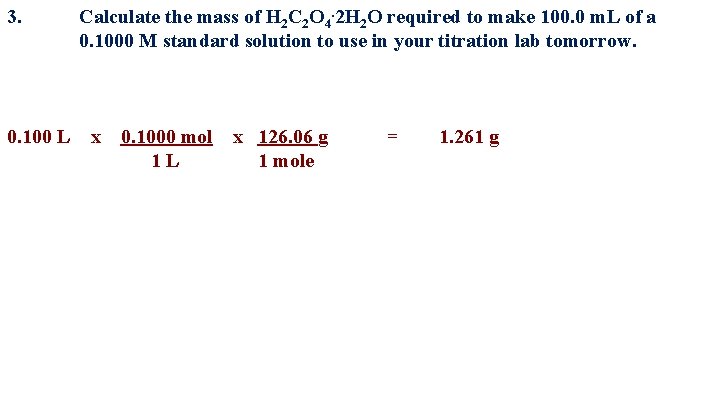
3. Calculate the mass of H 2 C 2 O 4. 2 H 2 O required to make 100. 0 m. L of a 0. 1000 M standard solution to use in your titration lab tomorrow. 0. 100 L x 0. 1000 mol 1 L x 126. 06 g 1 mole = 1. 261 g
- Slides: 9