Acid Base Reactions Chapter 4 part IV Characteristics

Acid- Base Reactions Chapter 4 part IV
Characteristics of acids l l l Sour Red litmus test Low p. H <7 Arrhenius: Produce H+ in water. Brømsted Lowry: produce protons. Lewis: Electron acceptor.

Characteristics of bases l l l Slippery & bitter Blue litmus test High p. H >7 Arrhenius: forms OHin water. Brømsted Lowry: accept protons. Lewis: Electron donor.

Net Ionic Equation l Of all strong acids plus strong bases: l H+ + OH- H 2 O l But what about weak acids and bases? l In a strong acid or base, it dissociates completely in water (strong electrolyte) l Weak acids and bases do not dissociate completely in water
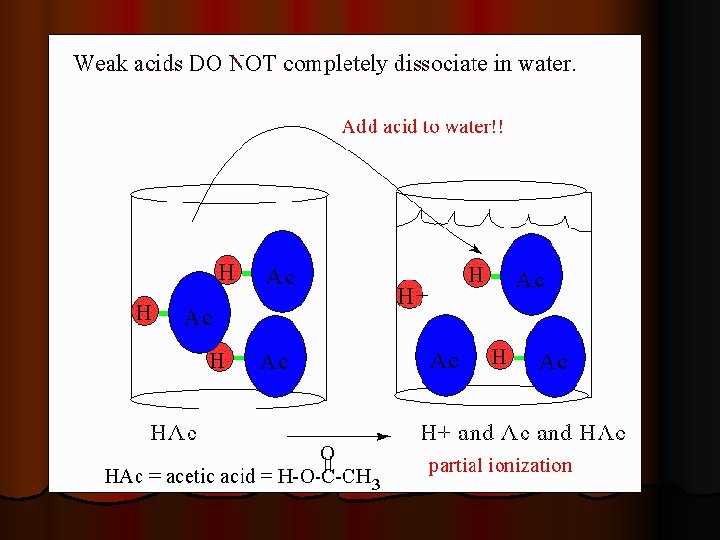

Weak electrolytes l In fact about 90% of acetic acid remains intact in aqueous solution. l Therefore the net ionic equation of a weak acid or base includes the intact weak acid or base. l Example: HC 2 H 3 O 2 +Na++ OH- H 2 O+ C 2 H 3 O 2 - +Na+ Net Ionic: HC 2 H 3 O 2 + OH- H 2 O + C 2 H 3 O 2 -
Weak electrolytes l An example of a weak base is NH 3. l So what is the net ionic equation for hydrochloric acid and ammonia? l HCl + NH 3 NH 4+ + Cll H+ + Cl- + NH 3 NH 4+ + Cll H+ + NH 3 NH 4+

Stoichiometry of an acid/base reaction: The steps. 1. 2. 3. 4. 5. List species present in the combined solution, before any reaction occurs. Decide what reaction will occur. Write out net ionic equation. Calculate moles of reactant in solution. Use volume of the original solution and its molarity. Determine the Limiting reagent.

Stoichiometry of an acid/base reaction: The steps. Calculate the moles of required reactant or product formed. 7. Convert to grams or volume as required. 8. In other words, after you answer the question, reread the question and make sure. 6.

Example: l What volume of 0. 1 M HCl solution is needed to neutralize 25. 0 m. L of 0. 350 M Na. OH?

Answer l List the species: H+ Cl- Na+ OHl What are the possible products? l Na. Cl (s) And H 2 O (l) l Na. Cl is soluble therefore it is not a possible product in solution. l Write the balanced equation. l H+(aq) + OH-(aq) H 2 O(l)

Answer l Calculate moles of reactants: l OH- = 25 m. L Na. OH x (1 L/1000 m. L)x (0. 350 mol OH-/L Na. OH) = l 8. 75 x 10 -3 mol OHl No limiting reagent, finding volume of HCl l Moles of reactant needed: molar ration is 1: 1 therefore need 8. 75 x 10 -3 mols acid. l Convert to volume: V x M=mol
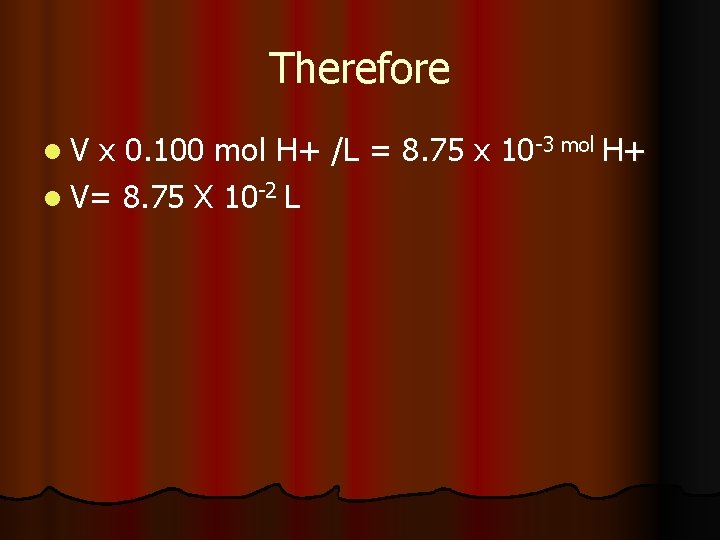
Therefore l. V x 0. 100 mol H+ /L = 8. 75 x 10 -3 mol H+ l V= 8. 75 X 10 -2 L
- Slides: 14