Acid Base Equilibria Types of Acids H vs

Acid Base Equilibria
![Types of Acids H+ vs H 3 O+ Arrhenius Definition Acids – Increase [H+] Types of Acids H+ vs H 3 O+ Arrhenius Definition Acids – Increase [H+]](http://slidetodoc.com/presentation_image_h/a883b808aa6f429f39caaa8c1618e777/image-2.jpg)
Types of Acids H+ vs H 3 O+ Arrhenius Definition Acids – Increase [H+] when dissolved in water Bases – Increase [OH-] when dissolved in water HCl Na. OH Brønsted-Lowry Definition Acids – Donates protons Bases – Accepts protons HCl(g) + H 2 O(l) → H 3 O+(aq) + Cl-(aq) HCl(g) + NH (g) → NH Cl(s)

Conjugate Acid Base Pairs HX(aq) + H 2 O(l) ↔ X-(aq) + H 3 O+(aq) What is the conjugate base of each of the following acids? HNO 2(aq) + H 2 O(l) ↔ NO 2 -(aq) + H 3 O+(aq) HCl. O 4, H 2 S, HCO 3 -, NH 4+ What is the conjugate acid of each of the following bases? CN-, SO 42 -, H 2 O, HCO 3 -

Practice There are two possible reactions that HSO 4 can have with water. Write the reaction in which it acts as an acid another where it acts as a base. When lithium oxide is dissolved in water, the solution turns basic from the reaction of the oxide ion(O 2 -) with water. Write the reaction and identify the conjugate acid base pairs.

Acid Base Strength Acid strength depends on how easily it gives up a proton What are the strong acids? Bases strength depends on how readily it accepts one. What are the strong bases?
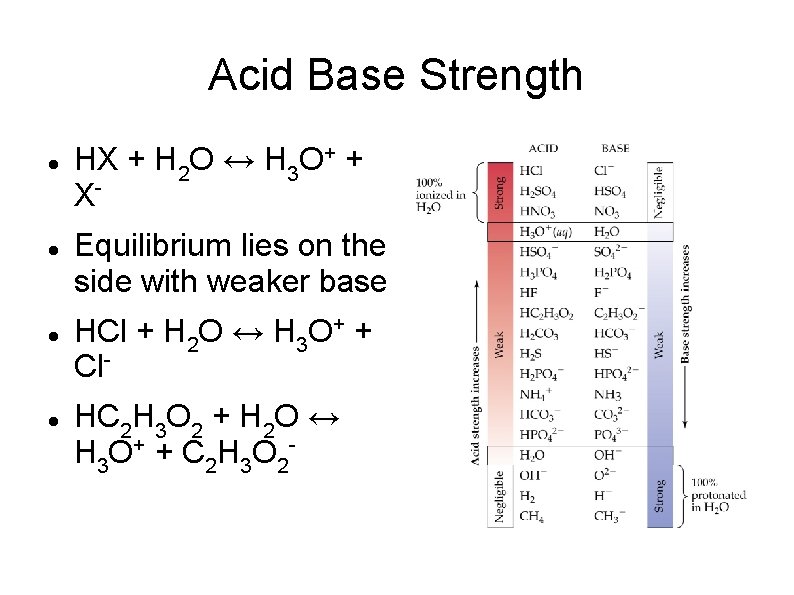
Acid Base Strength HX + H 2 O ↔ H 3 O+ + XEquilibrium lies on the side with weaker base HCl + H 2 O ↔ H 3 O+ + Cl. HC 2 H 3 O 2 + H 2 O ↔ H 3 O + + C 2 H 3 O 2 -

Practice Identify whether equilibrium lies predominantly to the left or right. HSO 4 - + CO 32 - ↔ SO 42 - + HCO 3 HPO 42 - + H 2 O ↔ H 2 PO 4 - + OHNH 4+ + OH- ↔ NH 3 + H 2 O Ans: R, L, R

Autoionization of Water H 2 O + H 2 O ↔ H 3 O+ + OH- What is the Ke q of this reaction At 25°C Ke q = 1 x 101 4 What are the concentrations of each ion if each the concentration of [H 3 O+]=[OH-]? How can we use this relationship to identify if a solution is acidic, basic, or neutral?
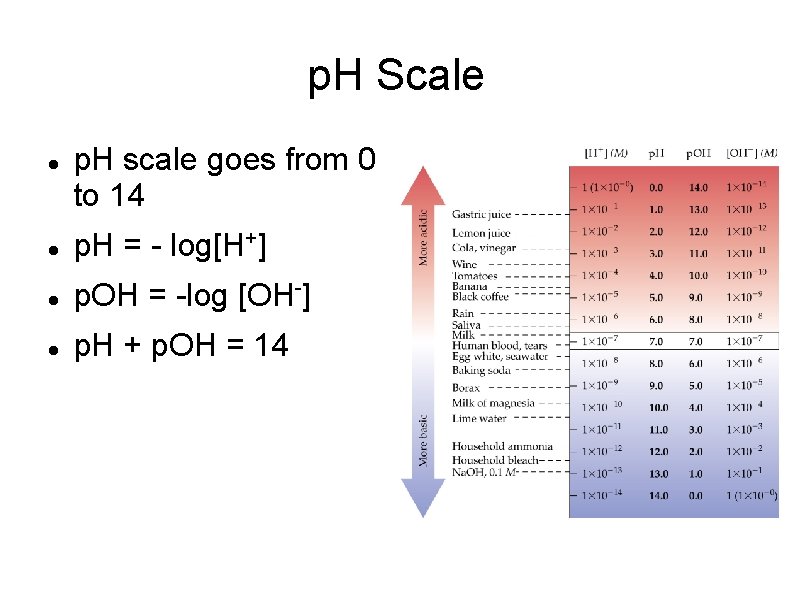
p. H Scale p. H scale goes from 0 to 14 p. H = - log[H+] p. OH = -log [OH-] p. H + p. OH = 14
Strong Acids and Bases For strong acids and bases no equilibrium is reached. For monoprotic strong acids: [H+] = [HA] What is the p. H of a 0. 040 M solution of HCl. O 4 p. H = -log(0. 040) = 1. 40 For strong bases: [OH-] = to # of OH-'s x [Base] What is the p. H of a 0. 028 M solution of Na. OH and a 0. 0011 M solution of Ca(OH)2
Practice An aqueous solution of HNO 3 has a p. H of 2. 34. What is the concentration of the acid? What is the concentration of a solution of KOH for which the p. H is 11. 89; Ca(OH)2 for which the p. H is 11. 68? Ans: #1 0. 0046 M, #2 7. 8 x 10 - 3 M, 2. 4 x 10 - 3 M
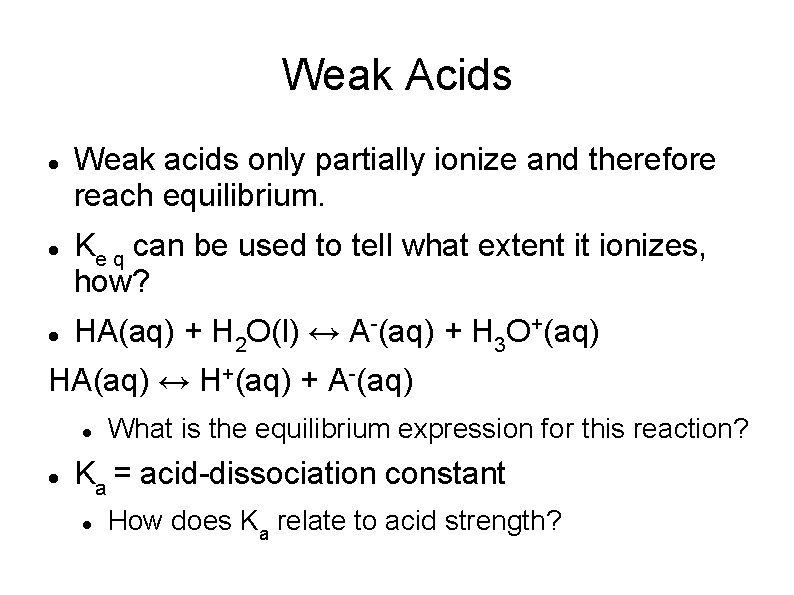
Weak Acids Weak acids only partially ionize and therefore reach equilibrium. Ke q can be used to tell what extent it ionizes, how? HA(aq) + H 2 O(l) ↔ A-(aq) + H 3 O+(aq) HA(aq) ↔ H+(aq) + A-(aq) What is the equilibrium expression for this reaction? Ka = acid-dissociation constant How does Ka relate to acid strength?
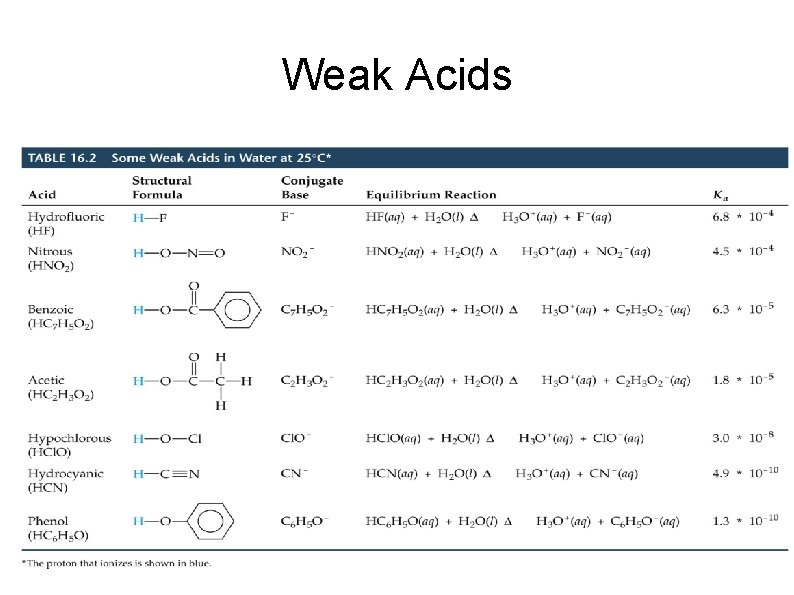
Weak Acids
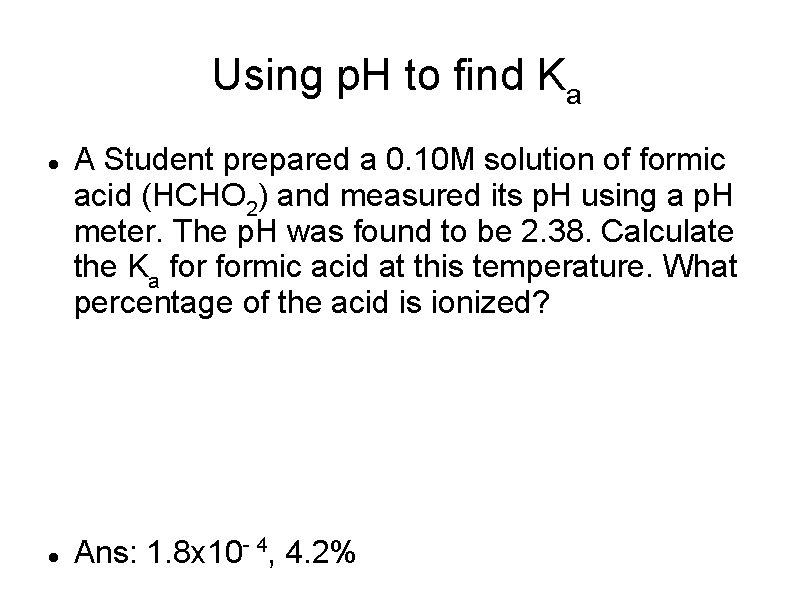
Using p. H to find Ka A Student prepared a 0. 10 M solution of formic acid (HCHO 2) and measured its p. H using a p. H meter. The p. H was found to be 2. 38. Calculate the Ka formic acid at this temperature. What percentage of the acid is ionized? Ans: 1. 8 x 10 - 4, 4. 2%
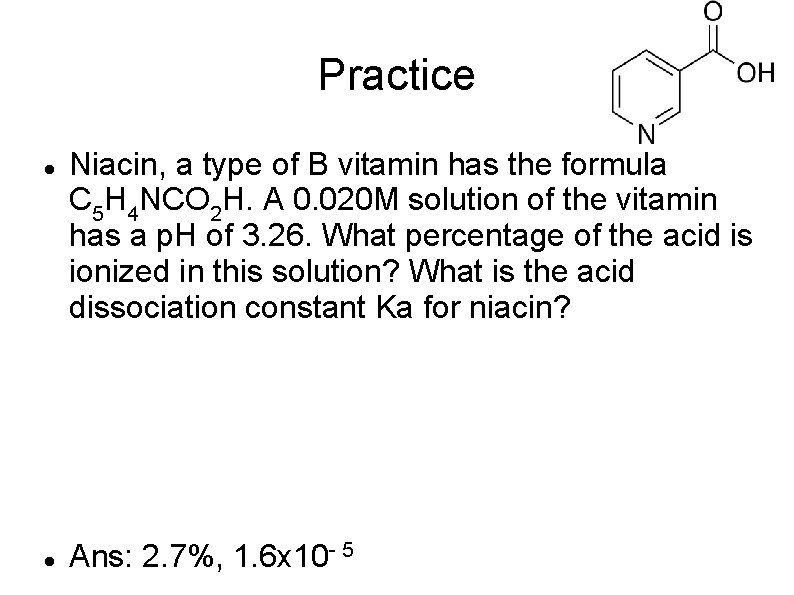
Practice Niacin, a type of B vitamin has the formula C 5 H 4 NCO 2 H. A 0. 020 M solution of the vitamin has a p. H of 3. 26. What percentage of the acid is ionized in this solution? What is the acid dissociation constant Ka for niacin? Ans: 2. 7%, 1. 6 x 10 - 5
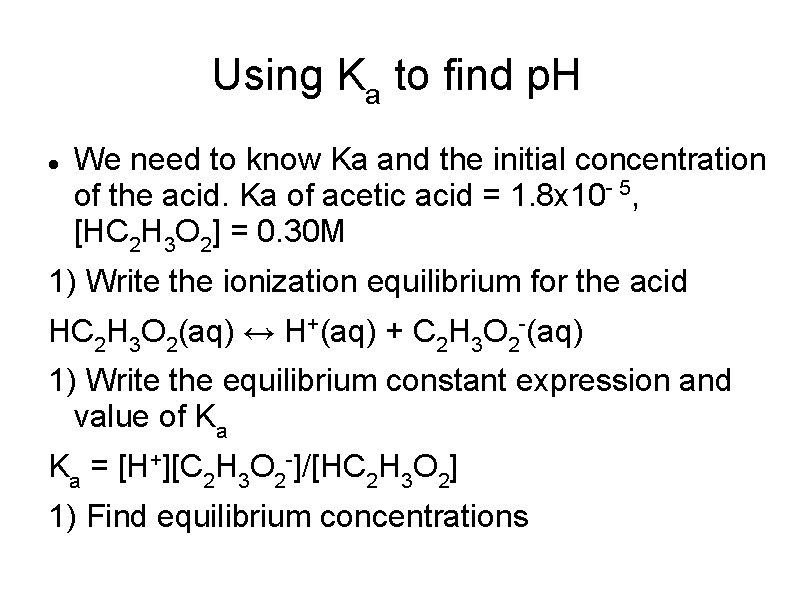
Using Ka to find p. H We need to know Ka and the initial concentration of the acid. Ka of acetic acid = 1. 8 x 10 - 5, [HC 2 H 3 O 2] = 0. 30 M 1) Write the ionization equilibrium for the acid HC 2 H 3 O 2(aq) ↔ H+(aq) + C 2 H 3 O 2 -(aq) 1) Write the equilibrium constant expression and value of Ka Ka = [H+][C 2 H 3 O 2 -]/[HC 2 H 3 O 2] 1) Find equilibrium concentrations

Using Ka to find p. H 4) Substitute the equilibrium concentrations into the equilibrium expression and solve for x. ** x may be disregarded as long as it is less than 5% of the initial [ ] x = 2. 3 x 10 - 3 = [H+] p. H = 2. 64

Practice Calculate the p. H of a 0. 20 M solution of HCN. Ka for HCN is 4. 9 x 10 - 1 0. Ans: p. H = 5. 00
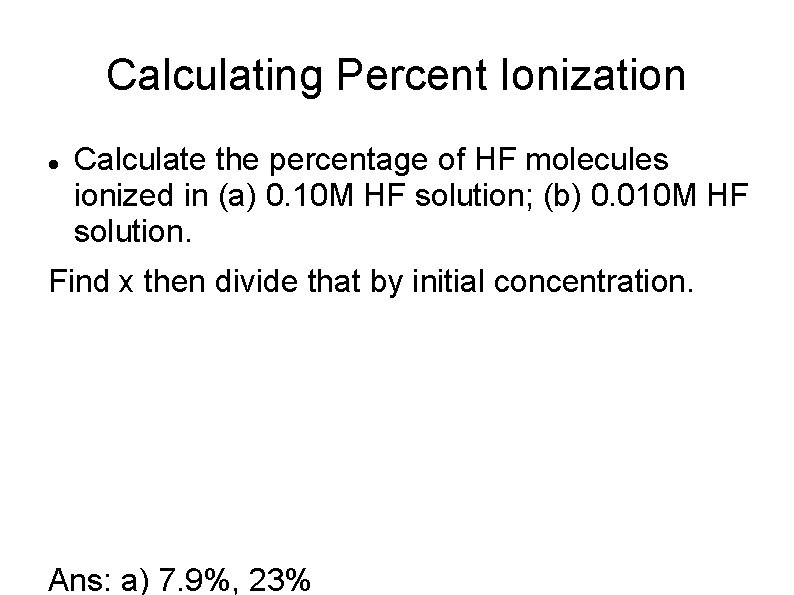
Calculating Percent Ionization Calculate the percentage of HF molecules ionized in (a) 0. 10 M HF solution; (b) 0. 010 M HF solution. Find x then divide that by initial concentration. Ans: a) 7. 9%, 23%
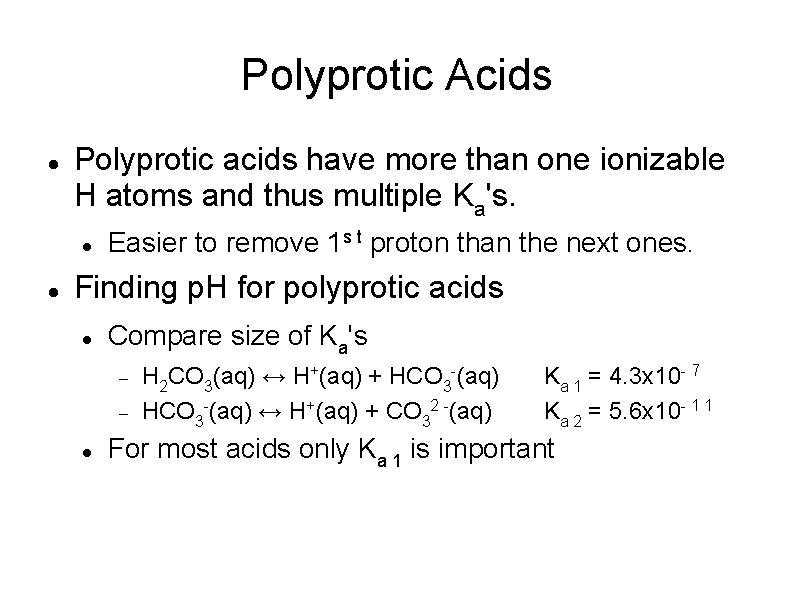
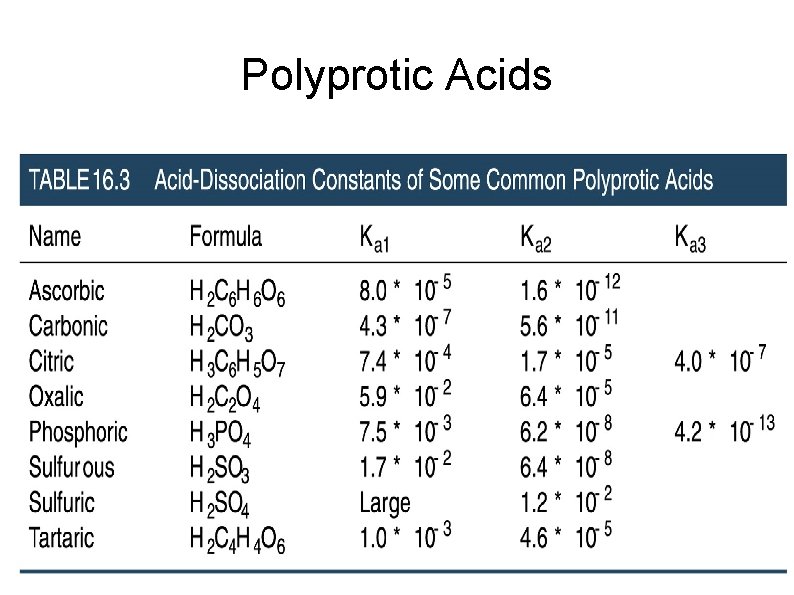
Polyprotic Acids Polyprotic acids have more than one ionizable H atoms and thus multiple Ka's. Easier to remove 1 s t proton than the next ones. Finding p. H for polyprotic acids Compare size of Ka's H 2 CO 3(aq) ↔ H+(aq) + HCO 3 -(aq) ↔ H+(aq) + CO 32 -(aq) Ka 1 = 4. 3 x 10 - 7 Ka 2 = 5. 6 x 10 - 1 1 For most acids only Ka 1 is important
Polyprotic Acids

p. H of Polyprotic Acids What is the p. H of a 0. 0037 M solution of H 2 CO 3? What is the [CO 32 -] in the solution? Ka -7, K -11 = 4. 3 x 10 = 5. 6 x 10 1 a 2 Ans: p. H = 4. 40, [CO 32 -] = 5. 6 x 10 - 1 1 M

Practice Calculate the p. H and concentration of oxalate ion [C 2 O 42 -], in a 0. 020 M solution of oxalic acid (H 2 C 2 O 4). Ka 1= 5. 9 x 10 - 2, Ka 2= 6. 4 x 10 - 5 Ans: p. H= 1. 80, [C 2 O 42 -]= 6. 4 x 10 - 5
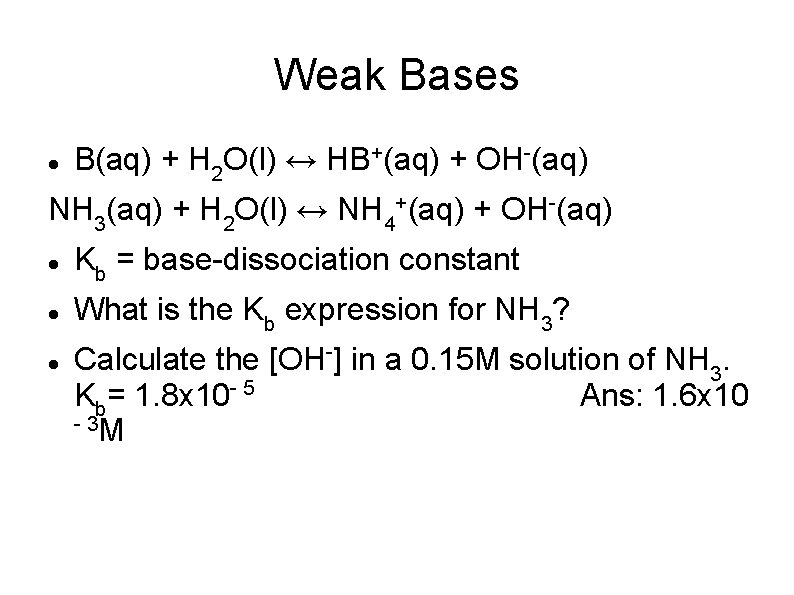
Weak Bases B(aq) + H 2 O(l) ↔ HB+(aq) + OH-(aq) NH 3(aq) + H 2 O(l) ↔ NH 4+(aq) + OH-(aq) Kb = base-dissociation constant What is the Kb expression for NH 3? Calculate the [OH-] in a 0. 15 M solution of NH 3. Kb= 1. 8 x 10 - 5 Ans: 1. 6 x 10 - 3 M
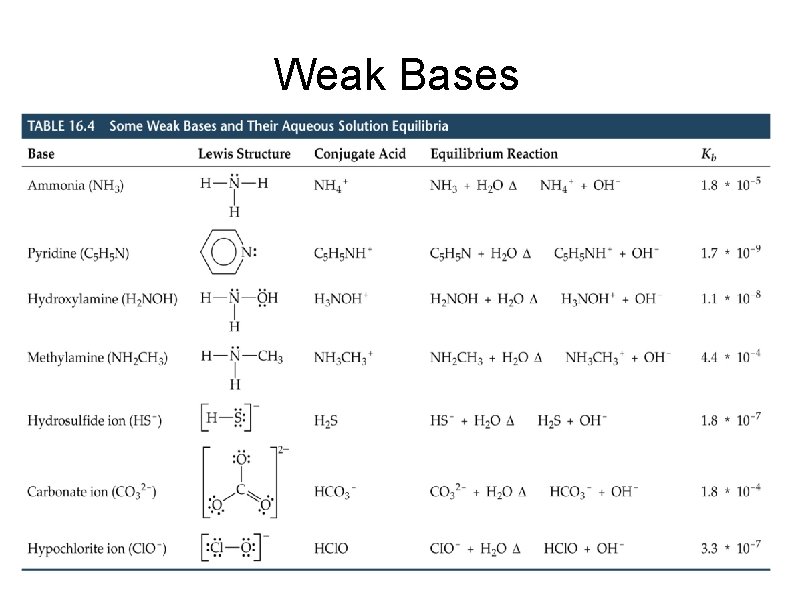
Weak Bases
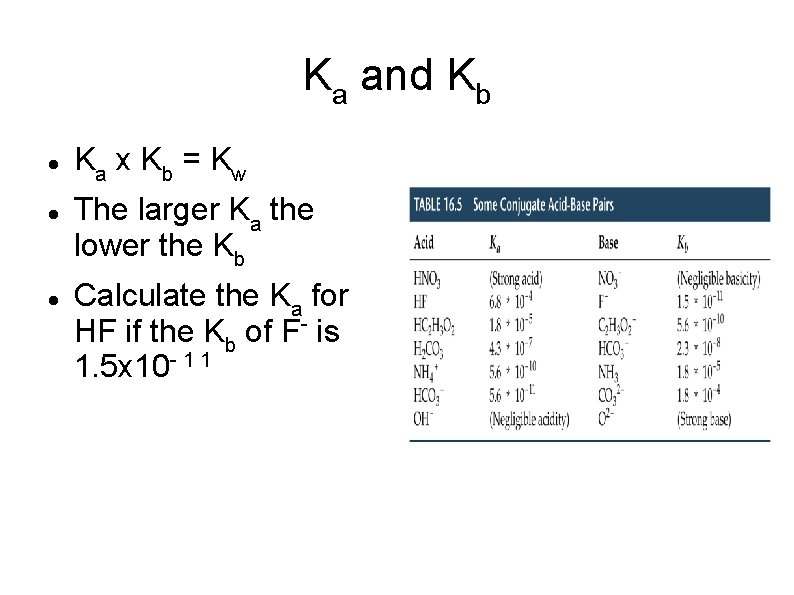
Ka and Kb Ka x K b = K w The larger Ka the lower the Kb Calculate the Ka for HF if the Kb of F- is 1. 5 x 10 - 1 1

p. H of Salt Solutions 1) An anion that is the conjugate base of a strong acid will not affect the p. H of a solution. ex. Br 2) An anion that is the conjugate base of a weak acid will cause an increase in p. H. ex. CN 3) A cation that is the conjugate acid of a weak base will cause a decrease in p. H. ex. NH 4+ 4) With the exception of ions of group 1 A and heavier members of group 2 A, metal ions will cause a decrease in p. H. 5) When a solution contains both the conjugate base of a weak acid and the conjugate acid of a
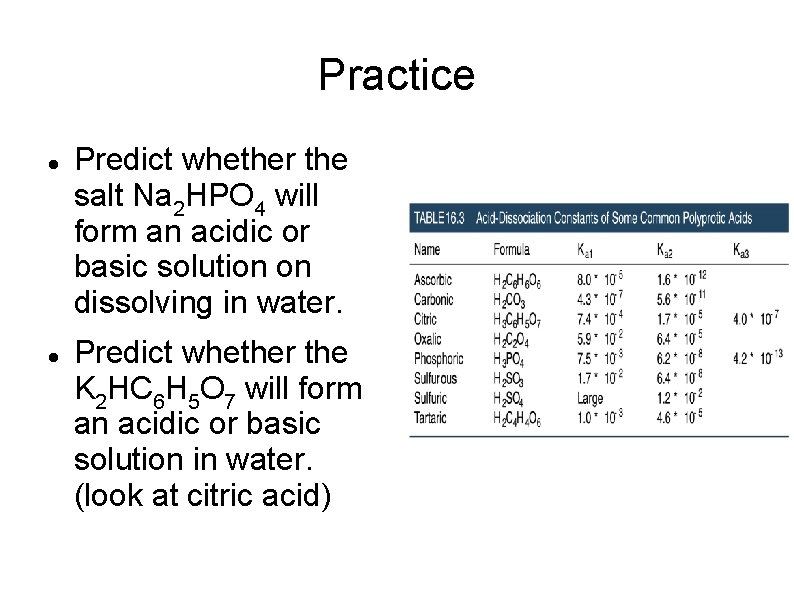
Practice Predict whether the salt Na 2 HPO 4 will form an acidic or basic solution on dissolving in water. Predict whether the K 2 HC 6 H 5 O 7 will form an acidic or basic solution in water. (look at citric acid)

Acid Strength Molecules only donate protons if the H—X bond is polar, where the anion X is more electronegative than the H. As you move from left to right X becomes more electronegative and acid strength increases. CH 4 < NH 3 << H 2 O < HF Strong H—X bonds are harder to break than weak ones. The strength of the H—X bond decreases as the size of X increases. HF versus HCl, H 2 S versus H 2 O
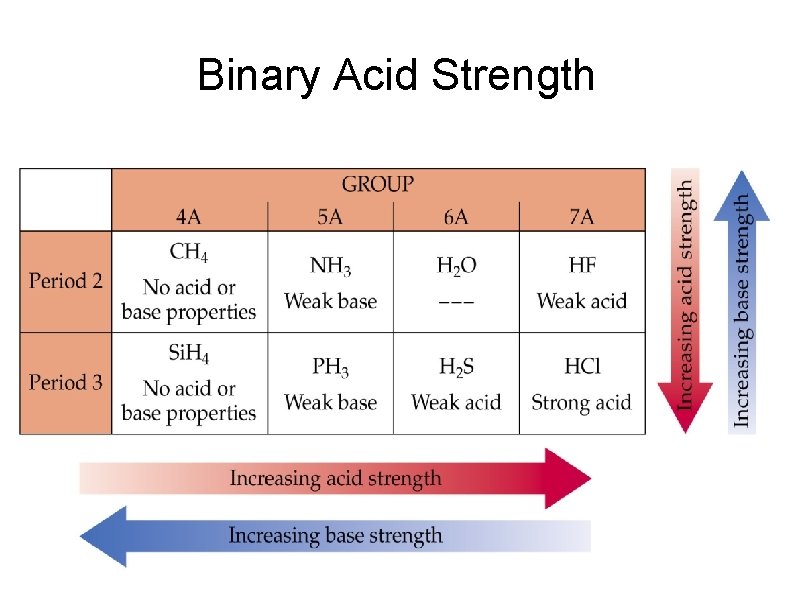
Binary Acid Strength
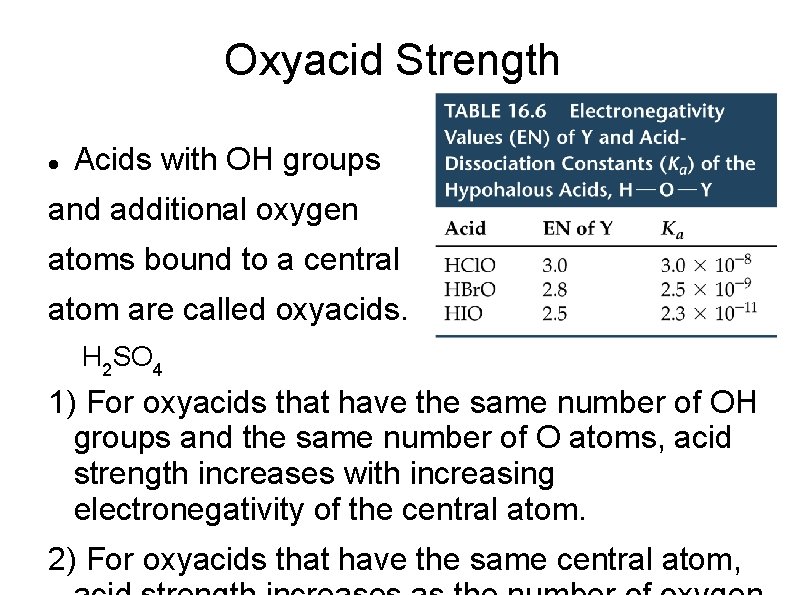
Oxyacid Strength Acids with OH groups and additional oxygen atoms bound to a central atom are called oxyacids. H 2 SO 4 1) For oxyacids that have the same number of OH groups and the same number of O atoms, acid strength increases with increasing electronegativity of the central atom. 2) For oxyacids that have the same central atom,
Lewis Acids and Bases Lewis acids – electron pair acceptor Lewis bases – electron pair donor Increases the types of compounds that we can consider acids All bases that are Brønsted-Lowry bases are Lewis bases. NH 3 + BF 3 → NH 3 BF 3 Metal ions reacting with water act as Lewis acids

Homework
- Slides: 33