Acid Base Equations General Form of Dissociation Acid

Acid Base Equations

General Form of Dissociation • • Acid HA H+ + Aor H 2 O + A H+ + AOHBase BOH B+ + OH- or H 2 O + B BH+ + OHStrong acids/bases will have a , weak acids/bases will have a ⇌

Weak acids and bases • • • Acids Carbonic acid Acetic acid Ammonium (CH 3 CH 2)3 NH+ Bases Carbonate Acetate Ammonia triethylamine (CH 3 CH 2)3 N
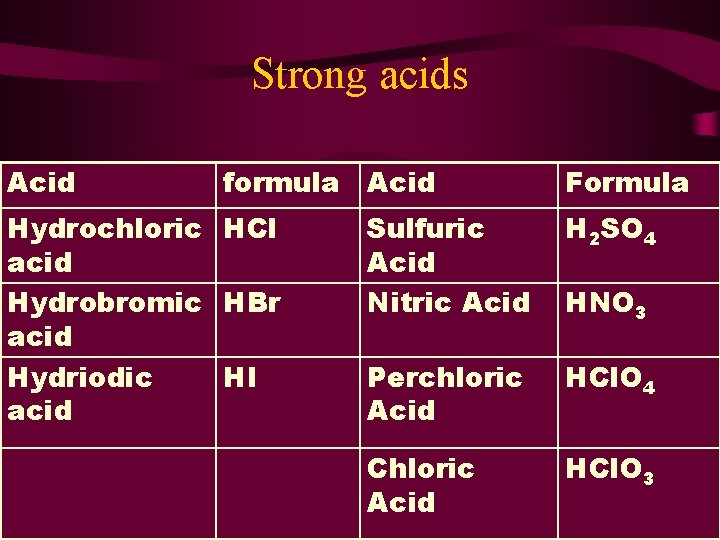
Strong acids Acid formula Acid Hydrochloric HCl acid Hydrobromic HBr acid Hydriodic HI acid Formula Sulfuric Acid Nitric Acid H 2 SO 4 Perchloric Acid HCl. O 4 Chloric Acid HCl. O 3 HNO 3

Strong Bases these make a lightning bolt on the periodic table! Name Formula Sodium Na. OH Hydroxide Calcium Ca(OH)2 Hydroxide Potassium KOH Hydroxide Strontium Sr(OH)2 Hydroxide Barium Ba(OH)2 Hydroxide

Strong acids and bases • Strong acids and bases are not at equilibrium, there is no reverse reaction. • Strong acids and bases will never be formed in a net ionic equation. • Adding strong acid or base normally means you will have H+ or OH- as a reactant, the rest is a spectator ion. • All other acids/bases can be formed by reacting the conjugate ion with a strong acid/base.

Weak + Strong reaction • Reacting an acid with a base will produce water. • Reacting a weak acid with a strong base will produce water and conjugate base. • H 2 CO 3 + OH- H 2 O + HCO 3 • Reacting a weak base with a strong acid will produce conjugate acid. • NH 3 + H+ NH 4+

Examples • • Calcium hydroxide reacts with chlorous acid Acetic acid reacts with potassium hydroxide Hydrochloric acid reacts with calcium nitrite Nitric acid reacts with sodium chlorite
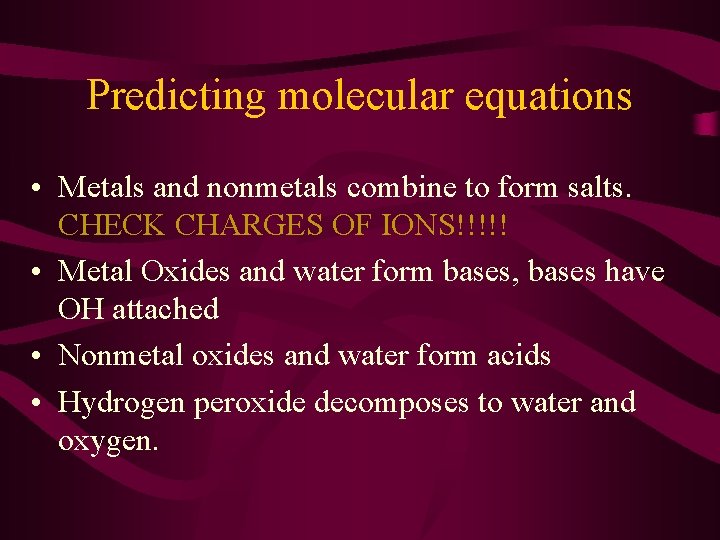
Predicting molecular equations • Metals and nonmetals combine to form salts. CHECK CHARGES OF IONS!!!!! • Metal Oxides and water form bases, bases have OH attached • Nonmetal oxides and water form acids • Hydrogen peroxide decomposes to water and oxygen.

Examples • Magnesium burns in oxygen • Calcium reacts with chlorine gas • Scandium oxide reacts with water • Carbon dioxide is bubbled through water • Hydrogen peroxide decomposes

Solubility Rules • You have to have this memorized • Acids are soluble. • Compounds of: alkali metals, ammonium, nitrate, chlorate and acetate are soluble. • This is the sheet I give you

Examples • Hydrochloric acid reacts with silver nitrate • Potassium carbonate reacts with calcium chlorate • Scandium acetate reacts with lithium chromate

Organic reactions • Combustion- organic compounds react with O 2 makes H 2 O + CO 2 • pentane is burned in air • C 5 H 12 +8 O 2 5 CO 2 + 6 H 2 O 13
- Slides: 13