ACID BASE BALANCE NUR 101 M Gardner Copyright

ACID BASE BALANCE NUR 101 M. Gardner Copyright 2/4/2013

ACID /BASE BALANCE �In order to meet homeostasis, the body fluids must maintain a stable chemical balance of hydrogen ions in body fluids. �This is done by regulating their acidity /alkalinity. �Deviation from a normal value indicates that the client is experiencing an acid/base imbalance
ACID/BASE BALANCE �ACID – substance that releases hydrogen ions (H+) �BASE �This – accept hydrogen ions in solution relationship is measured as p. H.
ABG’S �Measurement of ABGs involves analysis of several components: �p. H �PCO 2 �PO 2 �HCO 3
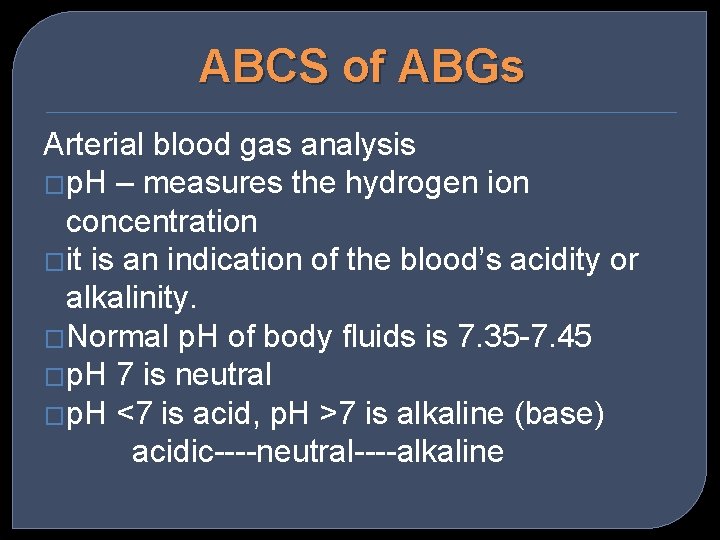
ABCS of ABGs Arterial blood gas analysis �p. H – measures the hydrogen ion concentration �it is an indication of the blood’s acidity or alkalinity. �Normal p. H of body fluids is 7. 35 -7. 45 �p. H 7 is neutral �p. H <7 is acid, p. H >7 is alkaline (base) acidic----neutral----alkaline

ABGs Pa. CO 2/PCO 2 35 -45 mm. Hg � Carbon dioxide/CO 2 � Reflects adequate ventilation by the lungs � Hyperventilation occurs Pa. CO 2 <35 mm. Hg. RR/depth increases the more carbon dioxide is exhaled � Hypoventilation occurs Pa. CO 2>45 mm. Hg. � RR/depth decreases, more carbon dioxide is retained – increasing the concentration of CO 2

ABGs HCO 3 /Bicarbonate � normal range 22 -26 m. E/L � base regulated by the kidneys � the kidneys excrete and retain HCO 3 to maintain a normal acid/base balance � is a principal buffer of the ECF compartment �< 22 m. Eq/L – indicates metabolic acidosis � >26 meq/L – indicates metabolic alkalosis

ABGs �PO 2 – oxygen in arterial blood �Normal range – 90 -100 mm. Hg
Regulation of Acid/Base Balance � Several body systems are actively involved in maintaining the narrow p. H range necessary for optimal function. � This includes buffers, respiratory system, renal system � Buffers maintain acid/base balance by neutralizing excess acids/bases � The lungs/kidneys help maintain a normal p. H by either excreting/retaining acid/bases.
BUFFERS �A strong acid added to the ECF causes the bicarbonate to become depleted neutralizing the acid p. H drops acidosis �A strong base is added to the ECF, depleting carbonic acid the p. H rises alkalosis �Buffer reaction is immediate

Respiratory Regulation �Lungs regulate acid/base balance by eliminating or retaining carbon dioxide (CO 2) �Carbon dioxide powerful stimulator of the respiratory center �CO 2 +H 2 O=H 2 CO 3 this reaction is reversible
Renal Regulation v Kidneys kick in by excreting or retaining bicarbonate and hydrogen ions. v Slower to respond to changes hour/days to correct imbalances
Renal Regulation �Excessive hydrogen ions are present and the p. H falls (acidosis) kidneys reabsorb bicarbonate & excrete hydrogen ions. �With alkalosis and high p. H excess bicarbonate is excreted and hydrogen ions are retained.
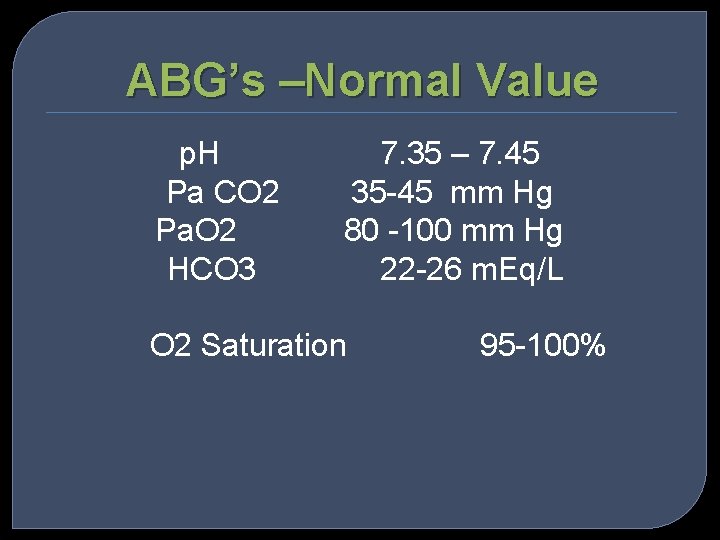
ABG’s –Normal Value p. H Pa CO 2 Pa. O 2 HCO 3 7. 35 – 7. 45 35 -45 mm Hg 80 -100 mm Hg 22 -26 m. Eq/L O 2 Saturation 95 -100%
Acid –Base Imbalances �Are classified as respiratory or metabolic considering the general/underlying cause of the disorder. �Respiratory acidosis/alkalosis retention/excretion of CO 2 �Bicarbonate /hydrogen levels are regulated by the kidneys, any problems metabolic acidosis/alkalosis
Respiratory Acidosis �Client hypoventilates CO 2 builds up in the bloodstream and the p. H drops below normal. �Kidneys try to compensate by conserving bicarbonate raises the p. H <7. 35 Pa. CO 2 >45 HCO 3 normal or elevated if compensating

Respiratory Acidosis Causes: �asthma, COPD �chest wall trauma �sedation medications �Acute lung conditions

Respiratory Acidosis Clinical Manifestations �apprehension �dizziness �muscular twitching �warm flushed skin �lethargy �diminished/absent breath sounds over the affected area

Respiratory Acidosis Interventions �bronchodilator �chest physiotherapy �suction �T, C, & DB �narcotic antagonist

Respiratory Alkalosis �Pt. hyperventilating this causes the lungs to blow off CO 2. ABG p. H > 7. 45 p. CO 2 <35 HCO 3 - normal or below 22, if compensating

Respiratory Alkalosis Causes Hyperventilation due to �extreme anxiety �pain �inappropriate mechanical ventilator settings �elevated body temperature

Respiratory Alkalosis Clinical Manifestations �increase in rate & depth of respirations �tachycardia �anxious, restlessness

Respiratory Alkalosis Interventions �treat the underlying disorder �allay anxiety – prevent hyperventilation �monitor VS �assist client to breathe in a paper bag
Metabolic Acidosis �Bicarbonate levels are low in relation to the amount of carbonic acid p. H low. ABG �p. H is below 7. 35 �p. CO 2 normal, if less than 35 may be compensated �HCO 3 -- <22 m. Eq/L

Metabolic Acidosis Causes �starvation �diarrhea �poisoning �diabetes

Metabolic Acidosis Clinical Manifestations �headache �lethargy �confusion �tachypnea with deep respirations

Metabolic Acidosis Interventions �treat the underlying problem �replace F/E �sodium bicarbonate – IV �monitor neurological status

Metabolic Alkalosis �Commonly associated with hypokalemia �Increase levels of bicarbonate ABG �p. H >7. 45 �p. CO 2 normal or above 45 if compensating �HCO 3 >26

Metabolic Alkalosis Cause �Excessive acid loss from the GI tract �Diuretic therapy

Metabolic Alkalosis Clinical manifestations �Slow, shallow respirations �S&S are commonly associated with an underlying condition

Metabolic Alkalosis Interventions �monitor VS �maintain patent IV access �monitor I&O �replace F&E
ABG Analysis �It is a respiratory problem if the p. H and CO 2 are traveling in the opposite directions. � p. H< 7. 35 & CO 2 >45 = Respiratory Acidosis � p. H >7. 35 & CO 2<35 = Respiratory Alkalosis
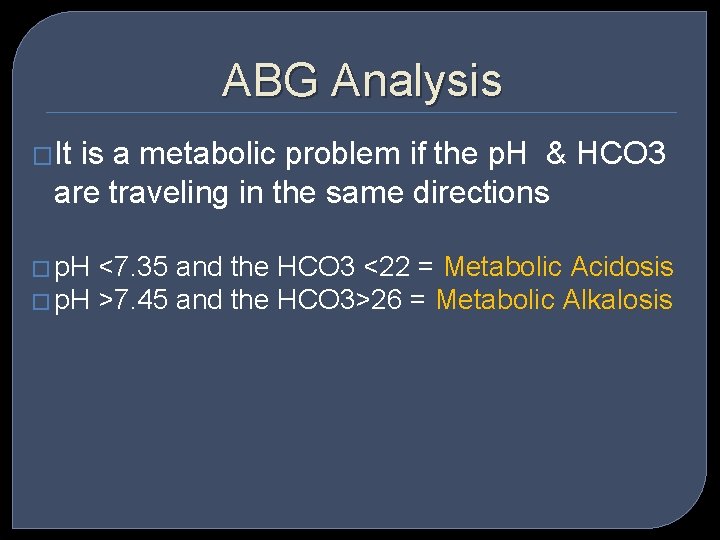
ABG Analysis �It is a metabolic problem if the p. H & HCO 3 are traveling in the same directions � p. H <7. 35 and the HCO 3 <22 = Metabolic Acidosis � p. H >7. 45 and the HCO 3>26 = Metabolic Alkalosis

Identify the Balance �p. H – 7. 30 �p. CO 2 – 36 mm. Hg �HCO 3 – 14 m. Eq/L �p. H – 7. 52 �p. CO 2 – 47 mm. Hg �HCO 3 – 43 m. Eq/L

THINK ABOUT THIS �The patient comes to the ER with complaint of vomiting for 3 days. Which acid base imbalance is she at risk for? �The patient has just returned from surgery. He was medicated twice with narcotic analgesics in the PACU. He is difficult to arouse and has a respiratory rate of 12. what acid/base imbalance is he at risk for?

RELAX �Some day you will know all of this!!!!
- Slides: 36