ACID BASE BALANCE Life is a struggle not

ACID BASE BALANCE Life is a struggle, not against sin, not against Money Power. . but against hydrogen ions. --H. L. Mencken
OVERVIEW OF DISCUSSION Basics of acid-base balance. Role of Renal/Respiratory system in acid-base homeostasis. Step-wise approach in diagnosis of acid-base disorders. Some practical examples
Acid Base Balance The body produces acids daily 15, 000 mmol CO 2 50 -100 m. Eq Nonvolatile acids The primary source is from metabolism of sulfur containing amino acids (cystine, methionine) and resultant formation of sulfuric acid. Other sources are non metabolized organic acids, phosphoric acid, lactic acid, citric acid. The lungs and kidneys attempt to maintain balance
Respiratory Regulation • 10 -12 mol/day CO 2 is accumulated and is transported to the lungs as Hb-generated HCO 3 and Hb-bound carbamino compounds where it is freely excreted. H 2 O + CO 2 ↔H 2 CO 3 ↔H+ + HCO 3 • Accumulation/loss of Co 2 changes p. H within minutes
Respiratory Regulation Balance affected by neurorespiratory control of ventilation. During Acidosis, chemoreceptors sense ↓p. H and trigger ventilation decreasing p. CO 2. Response to alkalosis is biphasic. Initial hyperventilation to remove excess p. CO 2 followed by suppression to increase p. CO 2 to return p. H to normal
Renal Regulation Kidneys are the ultimate defense against the addition of non-volatile acid/alkali Kidneys play a role in the maintenance of this HCO 3¯ by: – Conservation of filtered HCO 3 ¯ – Regeneration of HCO 3 ¯ Kidneys balance nonvolatile acid generation during metabolism by excreting acid.
Renal Regulation • Renal Excretion of acid – combining hydrogen ions with either urinary buffers to form titrable acid. eg: Phosphate, urate, ammonia
Acid Base Status • Assessment of status via bicarbonate-carbon dioxide buffer system in blood. – CO 2 + H 2 O <--> H 2 CO 3 <--> HCO 3 - + H+ – Henderson-Hasselbach equation – PH = 6. 10 + log ([HCO 3] / [0. 03 x PCO 2])

DEFINITIONS AND TERMINOLOGY 3 Component Terminology Acidosis/Alkalosis Respiratory/Metabolic Compensated/Uncompensated
Basic terminology • p. H – signifies free hydrogen ion concentration. p. H is inversely related to H+ ion concentration. • Acid – a substance that can donate H+ ion, i. e. lowers p. H. • Base –a substance that can accept H+ ion, i. e. raises p. H. • Anion – an ion with negative charge. • Cation – an ion with positive charge. • Acidemia – blood p. H< 7. 35 with increased H+ concentration. • Alkalemia – blood p. H>7. 45 with decreased H+ concentration. • Acidosis – Abnormal process or disease which reduces p. H due to increase in acid or decrease in alkali. • Alkalosis – Abnormal process or disease which increases p. H due to decrease in acid or increase in alkali.

Assessment of acid base balance ABG-: p. H, Pa. O 2, Pa. CO 2, Sa. O 2, HCO 3. Complete and objective overview of respiratory physiology

The pulse-oxymeter or saturation meter Non invasive measurement Finger probes and ear probes Percutaneous measurements
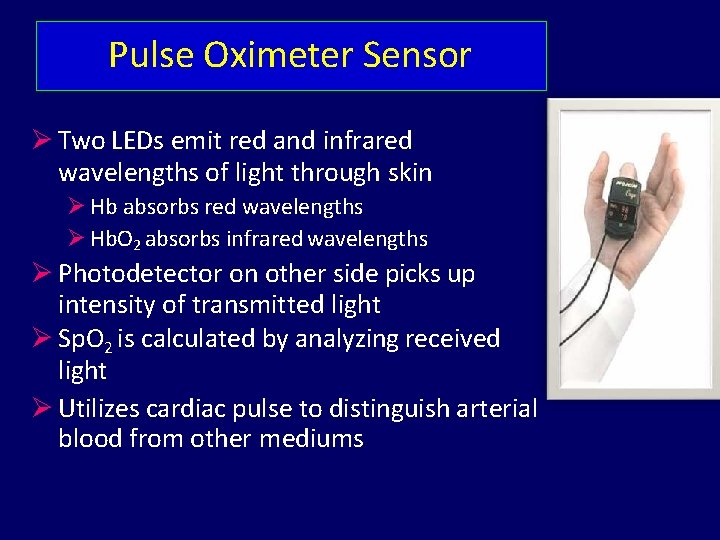
Pulse Oximeter Sensor Two LEDs emit red and infrared wavelengths of light through skin Hb absorbs red wavelengths Hb. O 2 absorbs infrared wavelengths Photodetector on other side picks up intensity of transmitted light Sp. O 2 is calculated by analyzing received light Utilizes cardiac pulse to distinguish arterial blood from other mediums
Pulse Oximetry Board Low power Data outputs: Sp. O 2 and pulse rate Eight second average (or instantaneous) Serial communication
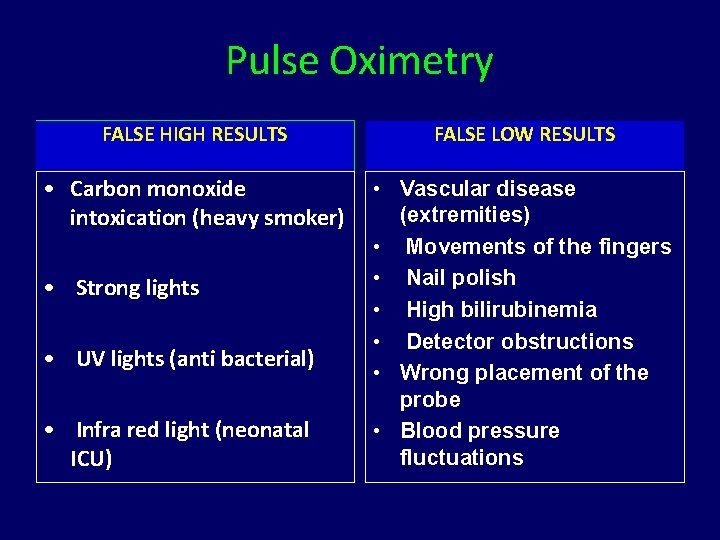
Pulse Oximetry FALSE HIGH RESULTS FALSE LOW RESULTS • Carbon monoxide intoxication (heavy smoker) • Vascular disease (extremities) • Movements of the fingers • Nail polish • High bilirubinemia • Detector obstructions • Wrong placement of the probe • Blood pressure fluctuations • Strong lights • UV lights (anti bacterial) • Infra red light (neonatal ICU)

Why Order an ABG? Aids in establishing a diagnosis Helps guide treatment plan Aids in ventilator management Improvement in acid/base management allows for optimal function of medications Acid/base status may alter electrolyte levels critical to patient status/care. Pre operative fitness.

Logistics • Where to place -- the options – – – Radial Femoral Brachial Dorsalis Pedis Axillary • When to order an arterial line -– Need for continuous BP monitoring – Need for multiple ABGs

Technical Errors • TYPE OF SYRINGE - Glass vs. plastic syringe: p. H & PCO 2 values unaffected PO 2 values drop more rapidly in plastic syringes (ONLY if PO 2 > 400 mm Hg) Other adv of glass syringes: Min friction of barrel with syringe wall Usually no need to ‘pull back’ barrel – less chance of air bubbles entering syringe Small air bubbles adhere to sides of plastic syringes – difficult to expel Though glass syringes preferred, differences usually not of clinical significance plastic syringes can be and continue to be used
Technical Errors • Excessive Heparin Dilutional effect on results HCO 3 - & Pa. CO 2 Syringe be emptied of heparin after flushing Risk of alteration of results with: 1) size of syringe/needle 2) vol of sample 25% lower values if 1 ml sample taken in 10 ml syringe (0. 25 ml heparin in needle) Syringes must be > 50% full with blood sample
Technical Errors Hyperventilation or Breathholding May lead to erroneous lab results Air bubbles PO 2 150 mm. Hg & PCO 2 0 mm Hg in air bubble. Mixing with sample lead to Pa. O 2 & Pa. CO 2 Mixing/Agitation diffusion more erroneous results Discard sample if excessive air bubbles Seal with cork/cap after taking sample Fever or Hypothermia Most ABG analyzers report data at N body temp If severe hyper/hypothermia, values of p. H & PCO 2 at 37 C can be significantly diff from pt’s actual values Changes in PO 2 values with temp predictable
Technical Errors Values other than p. H & PCO 2 do not change with temp Hansen JE, Clinics in Chest Med 10(2), 1989 227 -237 Some analysers calculate values at both 37 C and pt’s temp automatically if entered Pt’s temp should be mentioned while sending sample & lab should mention whether values being given in report at 37 C/pts actual temp

Technical Errors WBC COUNT 0. 1 ml of O 2 consumed/d. L of blood in 10 min in pts with N TLC Marked increase in pts with very high TLC/plt counts – hence chilling/analysis essential
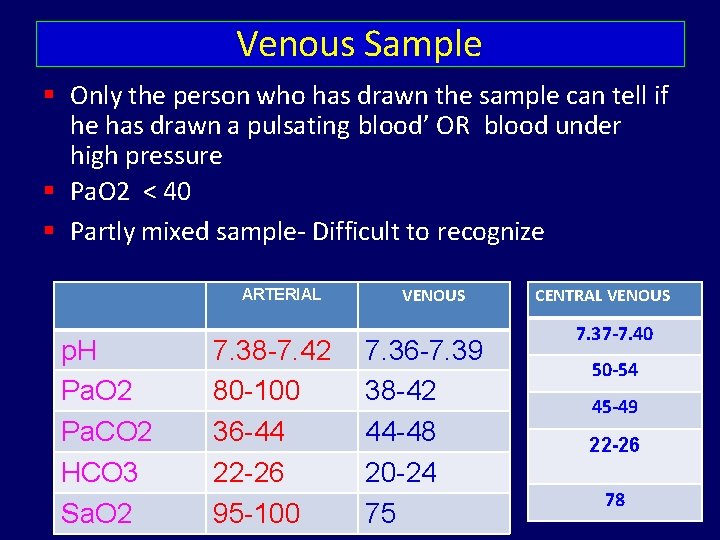
Venous Sample Only the person who has drawn the sample can tell if he has drawn a pulsating blood’ OR blood under high pressure Pa. O 2 < 40 Partly mixed sample- Difficult to recognize ARTERIAL p. H Pa. O 2 Pa. CO 2 HCO 3 Sa. O 2 7. 38 -7. 42 80 -100 36 -44 22 -26 95 -100 VENOUS 7. 36 -7. 39 38 -42 44 -48 20 -24 75 CENTRAL VENOUS 7. 37 -7. 40 50 -54 45 -49 22 -26 78
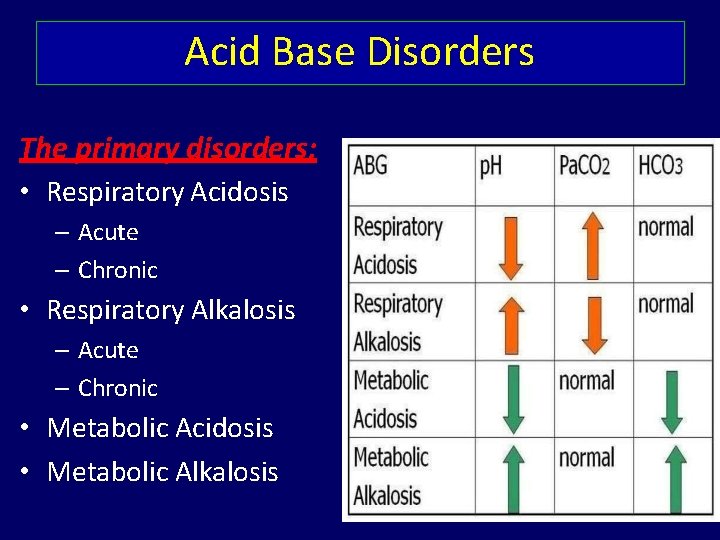
Acid Base Disorders The primary disorders: • Respiratory Acidosis – Acute – Chronic • Respiratory Alkalosis – Acute – Chronic • Metabolic Acidosis • Metabolic Alkalosis
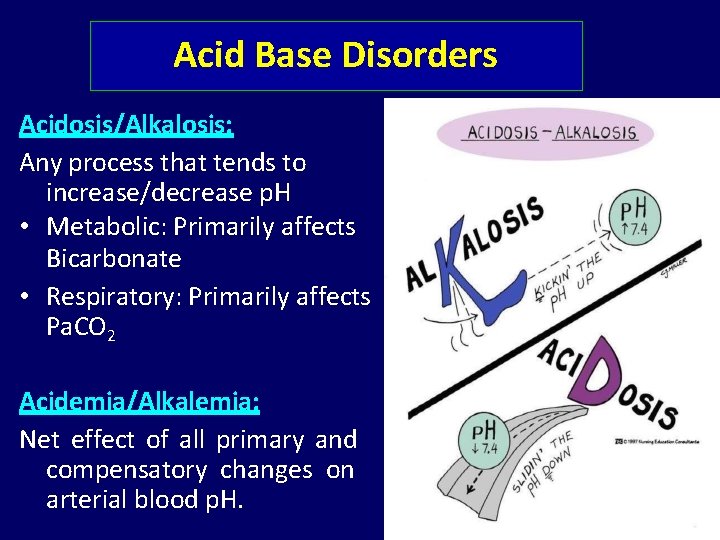
Acid Base Disorders Acidosis/Alkalosis: Any process that tends to increase/decrease p. H • Metabolic: Primarily affects Bicarbonate • Respiratory: Primarily affects Pa. CO 2 Acidemia/Alkalemia: Net effect of all primary and compensatory changes on arterial blood p. H.
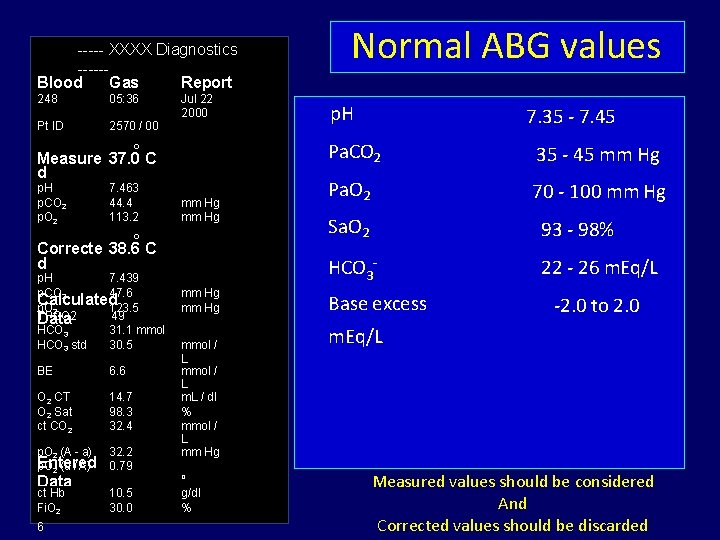
----- XXXX Diagnostics -----Blood Gas Report 248 05: 36 Pt ID 2570 / 00 Jul 22 2000 o Measure 37. 0 C d p. H p. CO 2 p. O 2 7. 463 44. 4 113. 2 mm Hg o Correcte 38. 6 C d p. H 7. 439 p. CO 2 47. 6 Calculated p. O 2 123. 5 TPCO 2 49 Data HCO 3 31. 1 mmol act 3 std /L HCO 30. 5 BE 6. 6 O 2 CT O 2 Sat ct CO 2 14. 7 98. 3 32. 4 p. O 2 (A - a) Entered p. O 2 (a / A) 32. 2 0. 79 ct Hb Temp Fi. O 2 10. 5 30. 0 38. Data 6 mm Hg mmol / L m. L / dl % mmol / L mm Hg o C g/dl % Normal ABG values p. H 7. 35 - 7. 45 Pa. CO 2 35 - 45 mm Hg Pa. O 2 70 - 100 mm Hg Sa. O 2 93 - 98% HCO 3¯ 22 - 26 m. Eq/L Base excess -2. 0 to 2. 0 m. Eq/L Measured values should be considered And Corrected values should be discarded

The ABG Interpretation Habits of Highly Successful Blood Gas Analysts
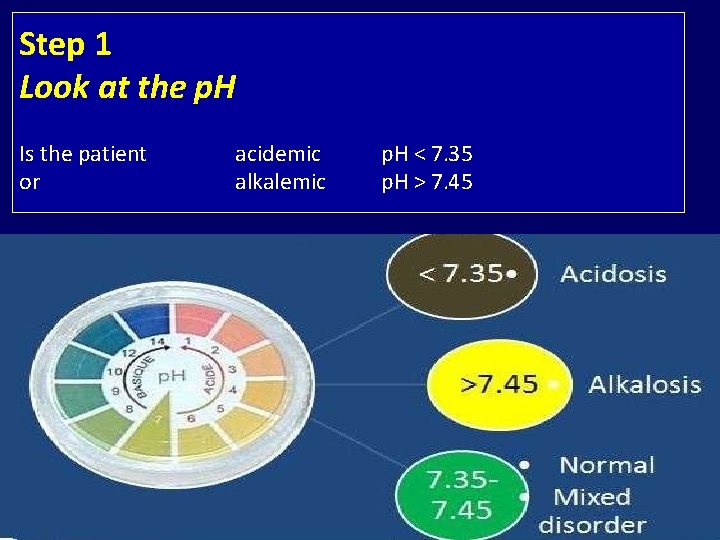
Step 1 Look at the p. H Is the patient or acidemic alkalemic p. H < 7. 35 p. H > 7. 45
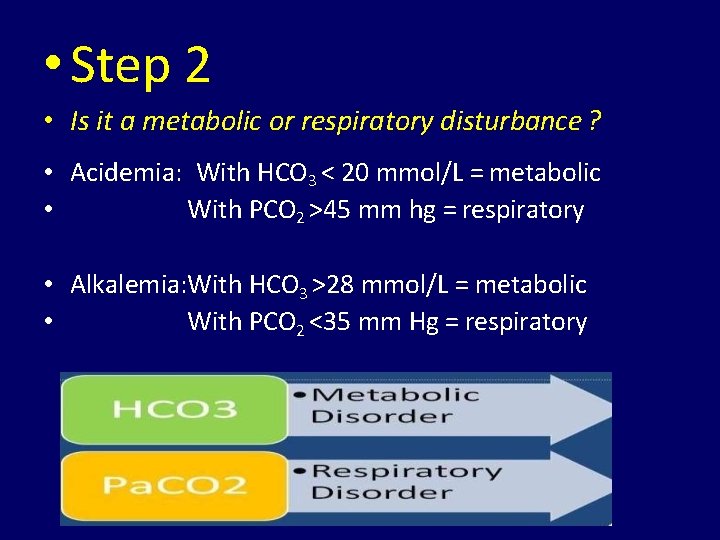
• Step 2 • Is it a metabolic or respiratory disturbance ? • Acidemia: With HCO 3 < 20 mmol/L = metabolic • With PCO 2 >45 mm hg = respiratory • Alkalemia: With HCO 3 >28 mmol/L = metabolic • With PCO 2 <35 mm Hg = respiratory
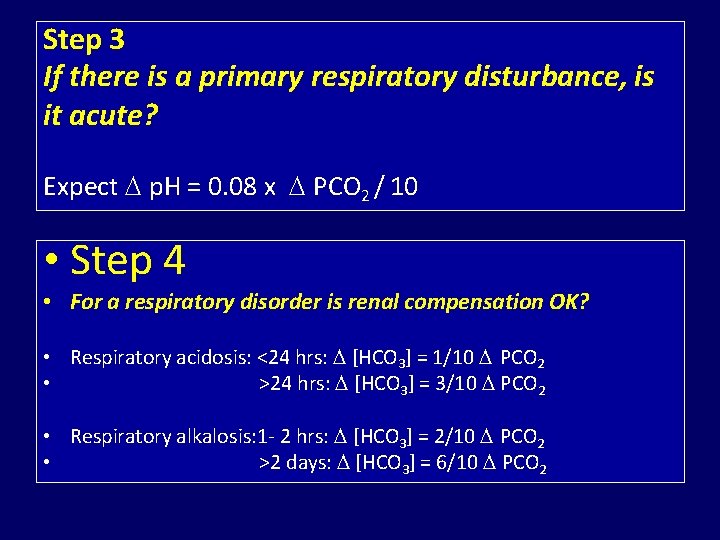
Step 3 If there is a primary respiratory disturbance, is it acute? Expect p. H = 0. 08 x PCO 2 / 10 • Step 4 • For a respiratory disorder is renal compensation OK? • Respiratory acidosis: <24 hrs: [HCO 3] = 1/10 PCO 2 • >24 hrs: [HCO 3] = 3/10 PCO 2 • Respiratory alkalosis: 1 - 2 hrs: [HCO 3] = 2/10 PCO 2 • >2 days: [HCO 3] = 6/10 PCO 2
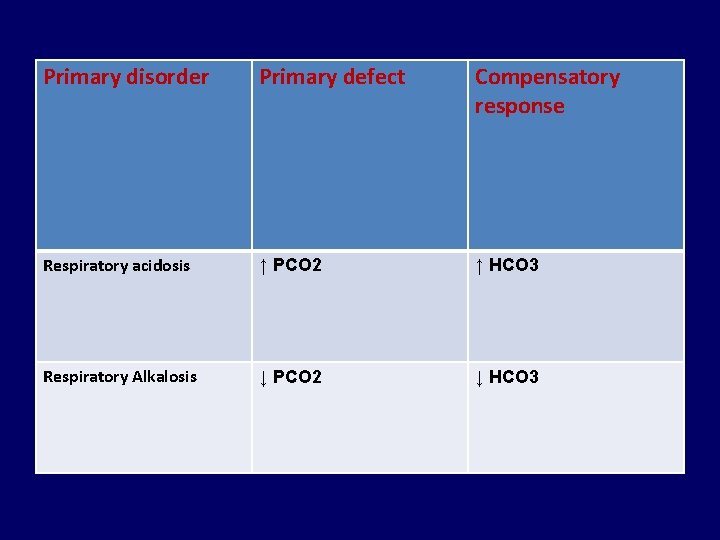
Primary disorder Primary defect Compensatory response Respiratory acidosis ↑ PCO 2 ↑ HCO 3 Respiratory Alkalosis ↓ PCO 2 ↓ HCO 3

• Step 5 • If the disturbance is metabolic is the respiratory compensation appropriate? • For metabolic acidosis: Expect PCO 2 = (1. 5 x [HCO 3]) + 8 + 2 • (Winter’s equation) • For metabolic alkalosis: • Expect PCO 2 = (0. 7 x [HCO 3]) + 21 + 1. 5 • If not: • actual PCO 2 > expected : hidden respiratory acidosis • actual PCO 2 < expected : hidden respiratory alkalosis
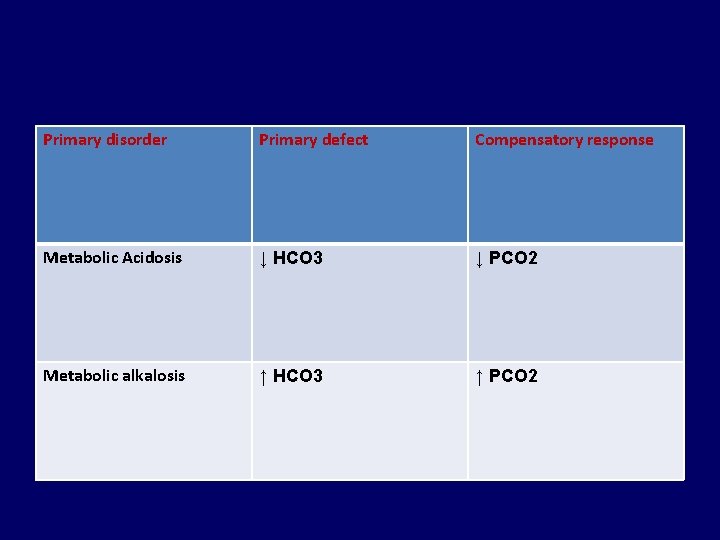
Primary disorder Primary defect Compensatory response Metabolic Acidosis ↓ HCO 3 ↓ PCO 2 Metabolic alkalosis ↑ HCO 3 ↑ PCO 2

During compensation HCO 3¯ & Pa. CO 2 move in the same direction

Respiratory compensati on is always FAST … 12 -24 hrs Metabolic compensation • is always SLOW. . . 5 -7 days

• Step 6 • If there is metabolic acidosis, is there an anion gap? • Na - (Cl-+ HCO 3 -) = Anion Gap usually <12 • Normal AG -: (loss of HC 03, increase in chloride) – Diarrhoea, RTA, carbonic anhydrase inhibitor use. • High AG-: If >12, Anion Gap Acidosis : Methanol Uremia • (Decreased excretion of acids) Diabetic Ketoacidosis • Paraldehyde • Infection (lactic acid) • Ethylene Glycol • Salicylate •

• Step 7 • Does the anion gap explain the change in bicarbonate? • (to rule out co-existence of 2 acid-base disorders) • anion gap (Anion gap -12) Delta Gap • Delta Gap + [HCO 3] = 22 -26 mmols/l • If Delta anion gap is greater(>26); consider additional metabolic alkalosis • If anion gap is less(<22); consider additional nonanion gap metabolic acidosis
RESPIRATORY ALKALOSIS
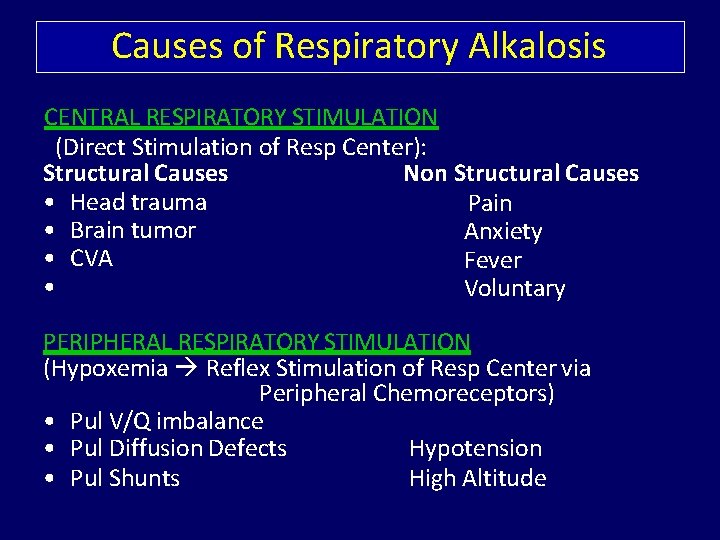
Causes of Respiratory Alkalosis CENTRAL RESPIRATORY STIMULATION (Direct Stimulation of Resp Center): Structural Causes Non Structural Causes • Head trauma Pain • Brain tumor Anxiety • CVA Fever • Voluntary PERIPHERAL RESPIRATORY STIMULATION (Hypoxemia Reflex Stimulation of Resp Center via Peripheral Chemoreceptors) • Pul V/Q imbalance • Pul Diffusion Defects Hypotension • Pul Shunts High Altitude

• 1. 2. 3. INTRATHORACIC STRUCTURAL CAUSES: Reduced movement of chest wall & diaphragm Reduced compliance of lungs Irritative lesions of conducting airways • 1. MIXED/UNKNOWN MECHANISMS: Nicotine Drugs – Salicylates Progesterone Thyroid hormone Catecholamines Xanthines (Aminophylline & related compounds) Cirrhosis Gram –ve Sepsis Pregnancy Heat exposure Mechanical Ventilation 2. 3. 4. 5. 6.

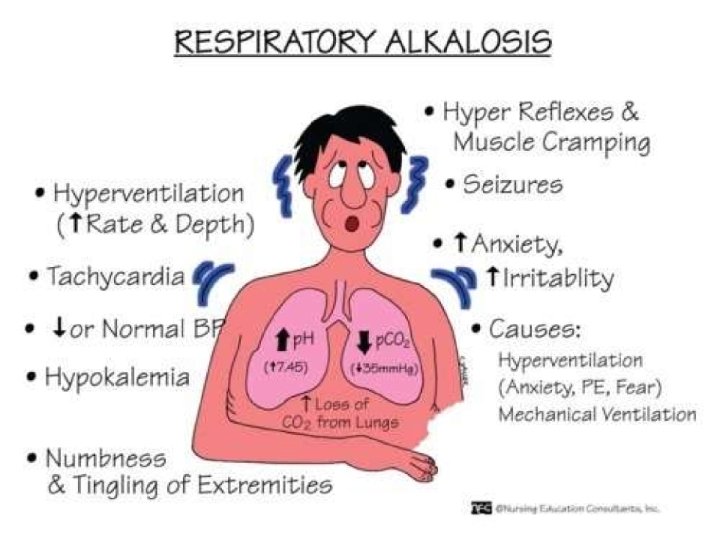
Manifestations of Resp Alkalosis • 1. 2. 3. 4. 5. 6. 7. 8. 9. NEUROMUSCULAR: Related to cerebral A vasoconstriction & Cerebral BF Lightheadedness Confusion Decreased intellectual function Syncope Seizures Paraesthesias (circumoral, extremities) Muscle twitching, cramps, tetany Hyperreflexia Strokes in pts with sickle cell disease
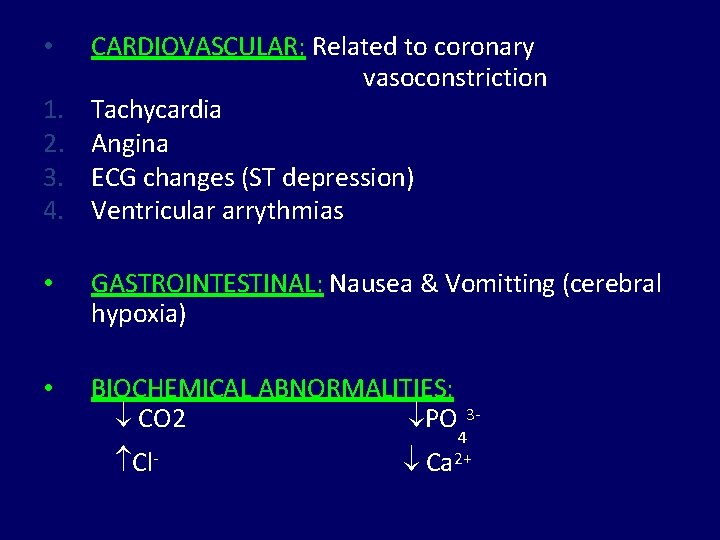
• 1. 2. 3. 4. CARDIOVASCULAR: Related to coronary vasoconstriction Tachycardia Angina ECG changes (ST depression) Ventricular arrythmias • GASTROINTESTINAL: Nausea & Vomitting (cerebral hypoxia) • BIOCHEMICAL ABNORMALITIES: CO 2 PO Cl- 34 Ca 2+

Homeostatic Response to Resp Alkalosis In ac resp alkalosis, imm response to fall in CO 2 (& H 2 CO 3) release of H+ by blood and tissue buffers react with HCO 3 - fall in HCO 3 - (usually not less than 18) and fall in p. H Cellular uptake of HCO 3 - in exchange for Cl Steady state in 15 min - persists for 6 hrs After 6 hrs kidneys increase excretion of HCO 3(usually not less than 12 -14) Steady state reached in 11/2 to 3 days. Timing of onset of hypocapnia usually not known except for pts on MV. Hence progression to subac and ch resp alkalosis indistinct in clinical practice

Treatment of Respiratory Alkalosis Resp alkalosis by itself not a cause of resp failure unless work of increased breathing not sustained by resp muscles. Rx underlying cause Usually extent of alkalemia produced not dangerous. Admn of O 2 if hypoxaemia If p. H>7. 55 pt may be sedated/anesthetised/ paralysed and/or put on MV.
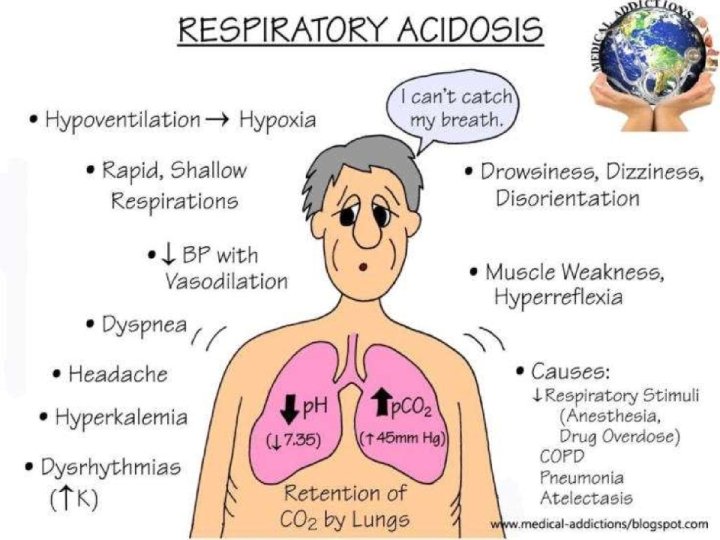
RESPIRATORY ACIDOSIS
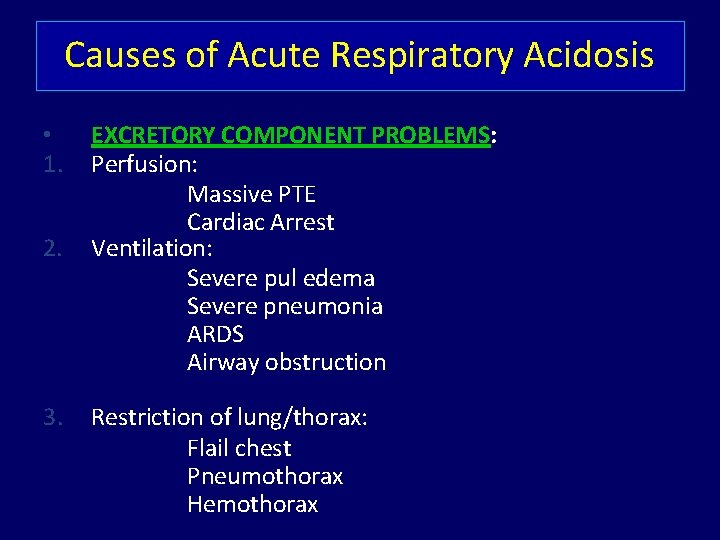
Causes of Acute Respiratory Acidosis • 1. 2. 3. EXCRETORY COMPONENT PROBLEMS: Perfusion: Massive PTE Cardiac Arrest Ventilation: Severe pul edema Severe pneumonia ARDS Airway obstruction Restriction of lung/thorax: Flail chest Pneumothorax Hemothorax

4. 5. Muscular defects: Severe hypokalemia Myasthenic crisis Failure of Mechanical Ventilator CONTROL COMPONENT PROBLEMS: 1. CNS: Drugs (Anesthetics, Sedatives) Trauma Stroke 2. Spinal Cord & Peripheral Nerves: Cervical Cord injury Neurotoxins (Botulism, Tetanus, OPC) Drugs causing Sk. m. paralysis (SCh, Curare, Pancuronium & allied drugs, aminoglycosides)
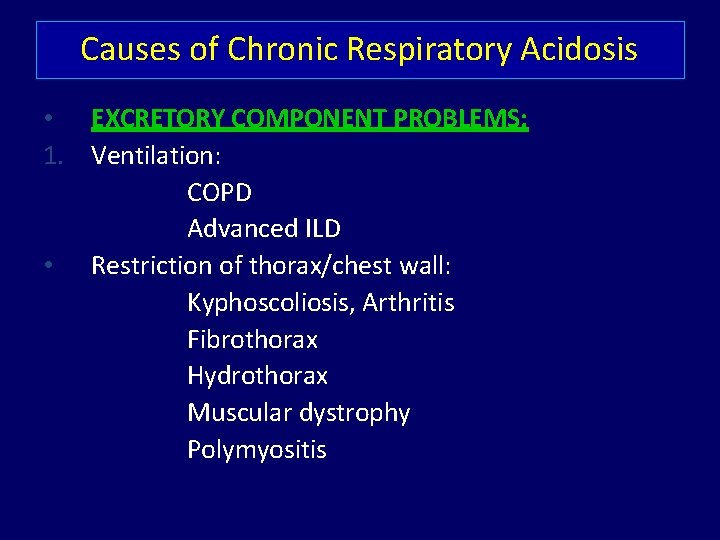
Causes of Chronic Respiratory Acidosis • EXCRETORY COMPONENT PROBLEMS: 1. Ventilation: COPD Advanced ILD • Restriction of thorax/chest wall: Kyphoscoliosis, Arthritis Fibrothorax Hydrothorax Muscular dystrophy Polymyositis
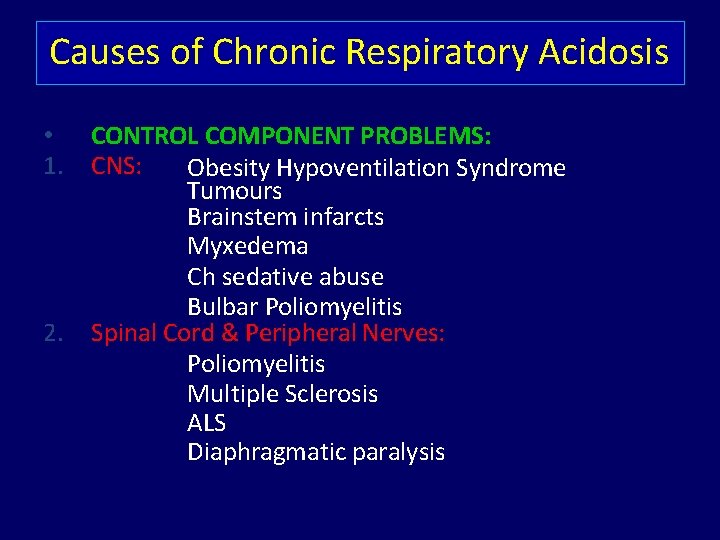
Causes of Chronic Respiratory Acidosis • 1. 2. CONTROL COMPONENT PROBLEMS: CNS: Obesity Hypoventilation Syndrome Tumours Brainstem infarcts Myxedema Ch sedative abuse Bulbar Poliomyelitis Spinal Cord & Peripheral Nerves: Poliomyelitis Multiple Sclerosis ALS Diaphragmatic paralysis

Manifestations of Resp Acidosis • NEUROMUSCULAR: Related to cerebral A vasodilatation & Cerebral BF 1. Anxiety 2. Asterixis 3. Lethargy, Stupor, Coma 4. Delirium 5. Seizures 6. Headache 7. Papilledema 8. Focal Paresis 9. Tremors, myoclonus
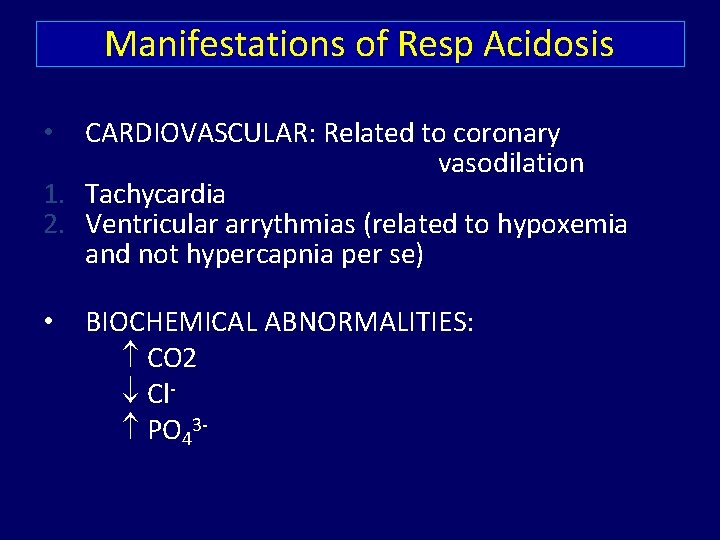
Manifestations of Resp Acidosis CARDIOVASCULAR: Related to coronary vasodilation 1. Tachycardia 2. Ventricular arrythmias (related to hypoxemia and not hypercapnia per se) • • BIOCHEMICAL ABNORMALITIES: CO 2 Cl PO 43 -

Homeostatic Response to Respiratory Acidosis Imm response to rise in CO 2 (& H 2 CO 3) blood and tissue buffers take up H+ ions, H 2 CO 3 dissociates and HCO 3 - increases with rise in p. H. Steady state reached in 10 min & lasts for 8 hours. PCO 2 of CSF changes rapidly to match Pa. CO 2. Hypercapnia that persists > few hours induces an increase in CSF HCO 3 - that reaches max by 24 hr and partly restores the CSF p. H. After 8 hrs, kidneys generate HCO 3 Steady state reached in 3 -5 d

Treatment of Respiratory Acidosis • Ensure adequate oxygenation care to avoid inadequate oxygenation while preventing worsening of hypercapnia due to supression of hypoxemic resp drive • Correct underlying disorder if possible

Treatment of Respiratory Acidosis Alkali (HCO 3) therapy rarely in ac and never in ch resp acidosis only if acidemia directly inhibiting cardiac functions Problems with alkali therapy: 1) Decreased alv ventilation by decrease in p. H mediated ventilatory drive 2)Enhanced carbon dioxide production from bicarbonate decomposition

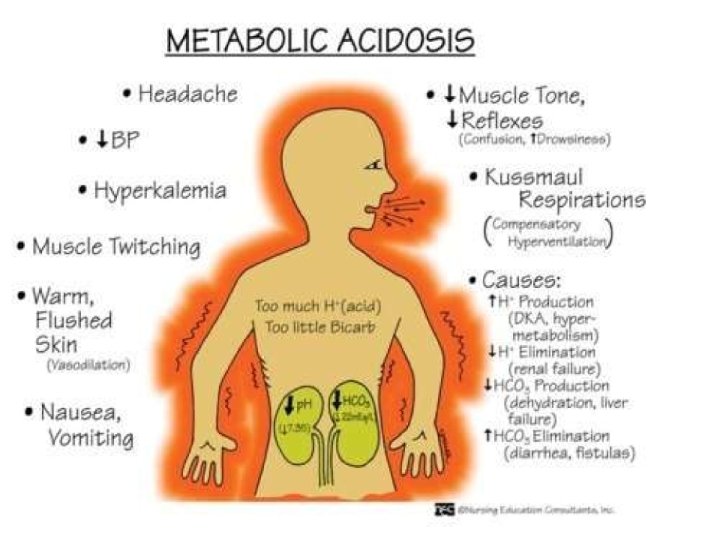
METABOLIC ACIDOSIS

Metabolic Acidosis • p. H, HCO 3 • 12 -24 hours for complete activation of respiratory compensation • PCO 2 by 1. 2 mm. Hg for every 1 m. Eq/L HCO 3 • The degree of compensation is assessed via the Winter’s Formula PCO 2 = 1. 5(HCO 3) +8 2
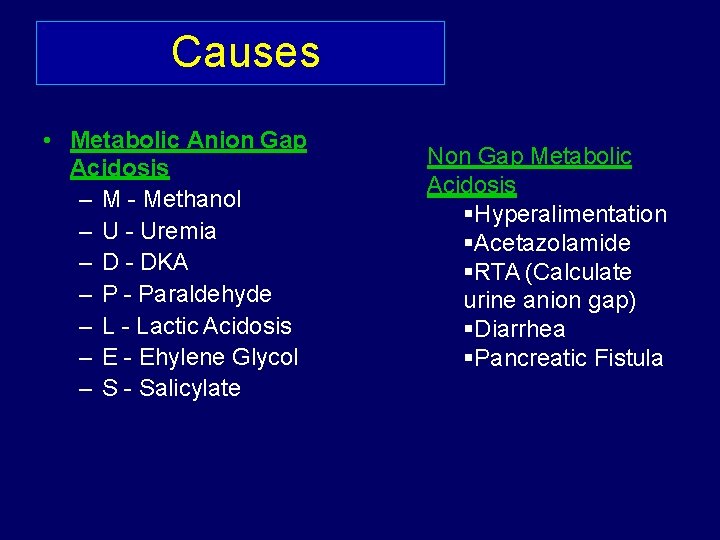
Causes • Metabolic Anion Gap Acidosis – M - Methanol – U - Uremia – D - DKA – P - Paraldehyde – L - Lactic Acidosis – E - Ehylene Glycol – S - Salicylate Non Gap Metabolic Acidosis Hyperalimentation Acetazolamide RTA (Calculate urine anion gap) Diarrhea Pancreatic Fistula
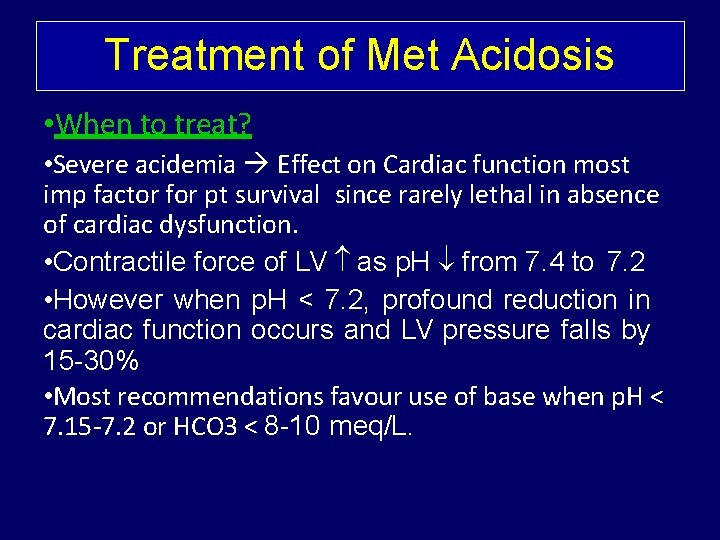
Treatment of Met Acidosis • When to treat? • Severe acidemia Effect on Cardiac function most imp factor for pt survival since rarely lethal in absence of cardiac dysfunction. • Contractile force of LV as p. H from 7. 4 to 7. 2 • However when p. H < 7. 2, profound reduction in cardiac function occurs and LV pressure falls by 15 -30% • Most recommendations favour use of base when p. H < 7. 15 -7. 2 or HCO 3 < 8 -10 meq/L.

How to treat? Rx Undelying Cause HCO 3 - Therapy • Aim to bring up p. H to 7. 2 & HCO 3 - 10 meq/L • Qty of HCO 3 admn calculated: 0. 5 x LBW (kg) x HCO 3 Deficity (meq/L)

Why not to treat? Considered cornerstone of therapy of severe acidemia for >100 yrs Based on assumption that HCO 3 - admn would normalize ECF & ICF p. H and reverse deleterious effects of acidemia on organ function However later studies contradicted above observations and showed little or no benefit from rapid and complete/over correction of acidemia with HCO 3.
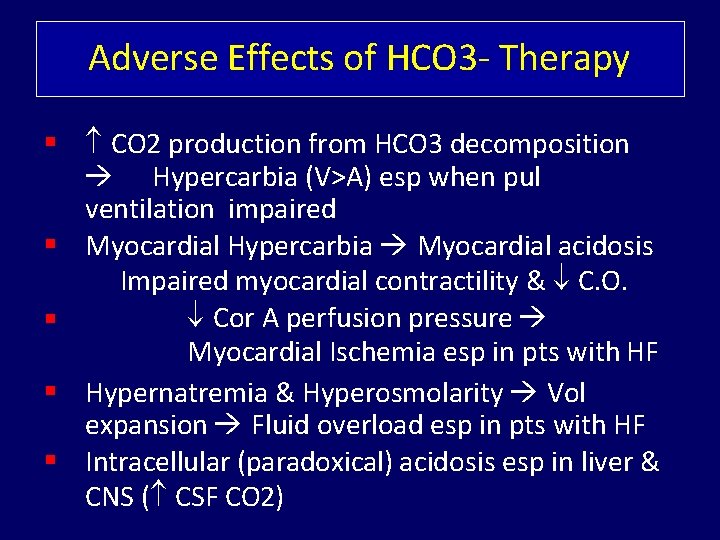
Adverse Effects of HCO 3 - Therapy CO 2 production from HCO 3 decomposition Hypercarbia (V>A) esp when pul ventilation impaired Myocardial Hypercarbia Myocardial acidosis Impaired myocardial contractility & C. O. Cor A perfusion pressure Myocardial Ischemia esp in pts with HF Hypernatremia & Hyperosmolarity Vol expansion Fluid overload esp in pts with HF Intracellular (paradoxical) acidosis esp in liver & CNS ( CSF CO 2)
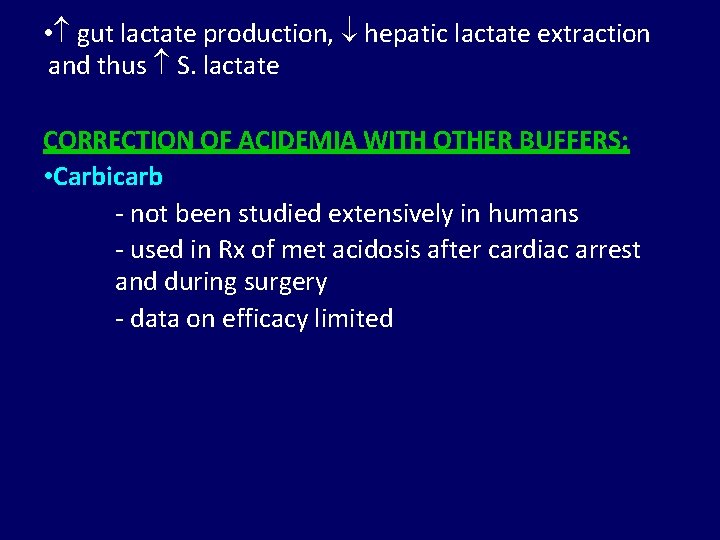
• gut lactate production, hepatic lactate extraction and thus S. lactate CORRECTION OF ACIDEMIA WITH OTHER BUFFERS: • Carbicarb - not been studied extensively in humans - used in Rx of met acidosis after cardiac arrest and during surgery - data on efficacy limited
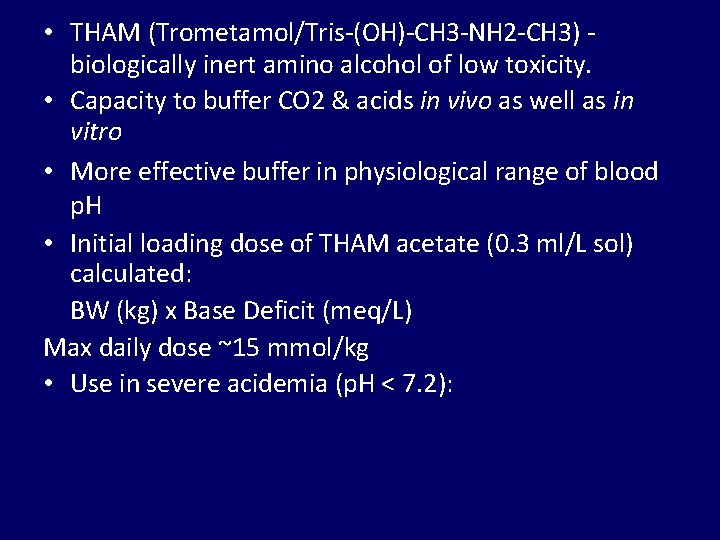
• THAM (Trometamol/Tris-(OH)-CH 3 -NH 2 -CH 3) biologically inert amino alcohol of low toxicity. • Capacity to buffer CO 2 & acids in vivo as well as in vitro • More effective buffer in physiological range of blood p. H • Initial loading dose of THAM acetate (0. 3 ml/L sol) calculated: BW (kg) x Base Deficit (meq/L) Max daily dose ~15 mmol/kg • Use in severe acidemia (p. H < 7. 2):

METABOLIC ALKALOSIS
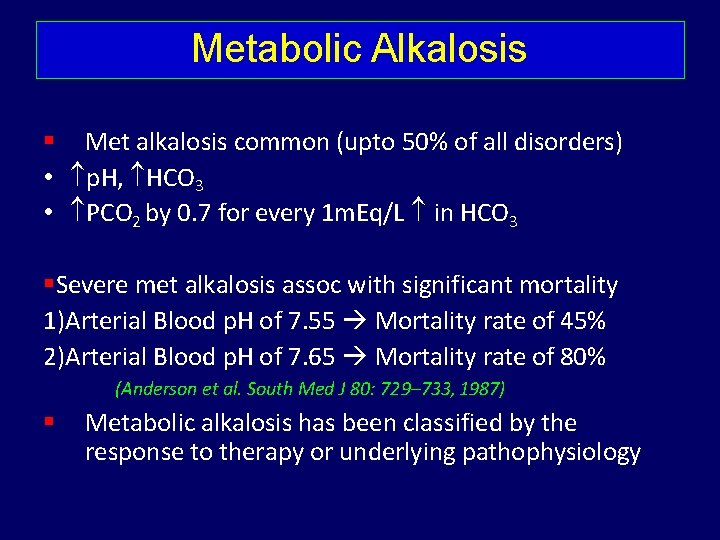
Metabolic Alkalosis Met alkalosis common (upto 50% of all disorders) • p. H, HCO 3 • PCO 2 by 0. 7 for every 1 m. Eq/L in HCO 3 Severe met alkalosis assoc with significant mortality 1)Arterial Blood p. H of 7. 55 Mortality rate of 45% 2)Arterial Blood p. H of 7. 65 Mortality rate of 80% (Anderson et al. South Med J 80: 729– 733, 1987) Metabolic alkalosis has been classified by the response to therapy or underlying pathophysiology
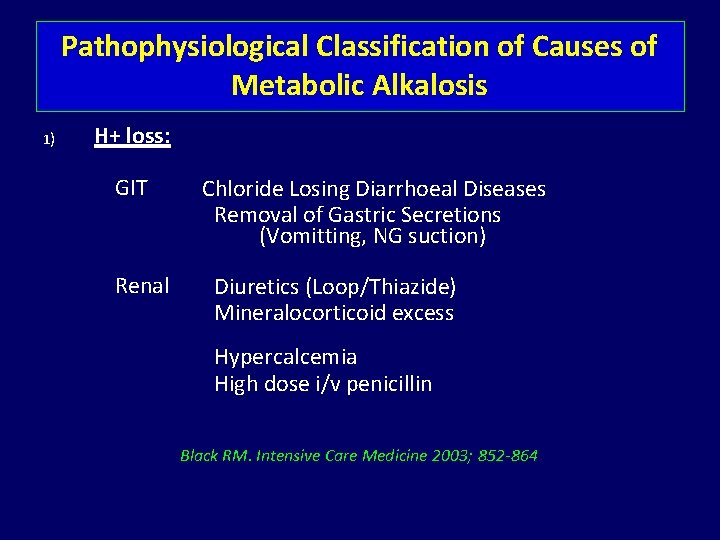
Pathophysiological Classification of Causes of Metabolic Alkalosis 1) H+ loss: GIT Renal Chloride Losing Diarrhoeal Diseases Removal of Gastric Secretions (Vomitting, NG suction) Diuretics (Loop/Thiazide) Mineralocorticoid excess Hypercalcemia High dose i/v penicillin Black RM. Intensive Care Medicine 2003; 852 -864
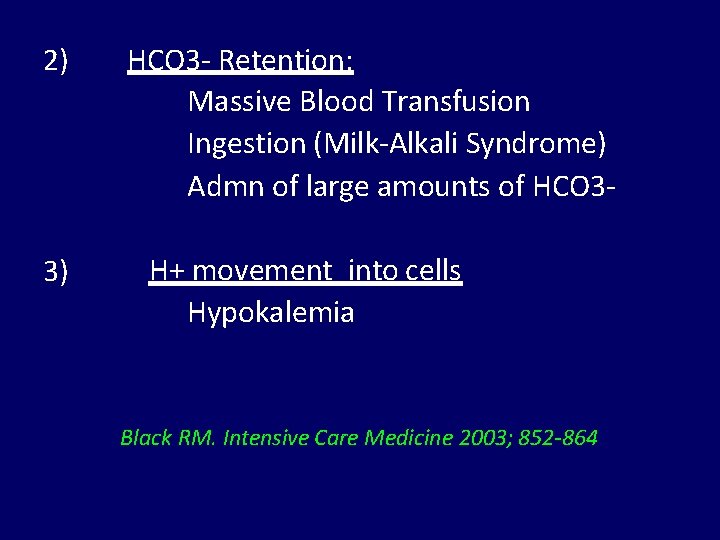
2) 3) HCO 3 - Retention: Massive Blood Transfusion Ingestion (Milk-Alkali Syndrome) Admn of large amounts of HCO 3 H+ movement into cells Hypokalemia Black RM. Intensive Care Medicine 2003; 852 -864
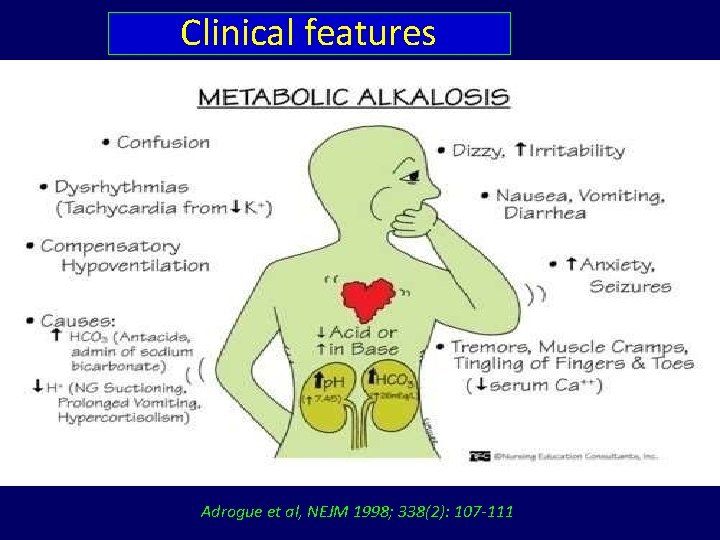
Clinical features Adrogue et al, NEJM 1998; 338(2): 107 -111
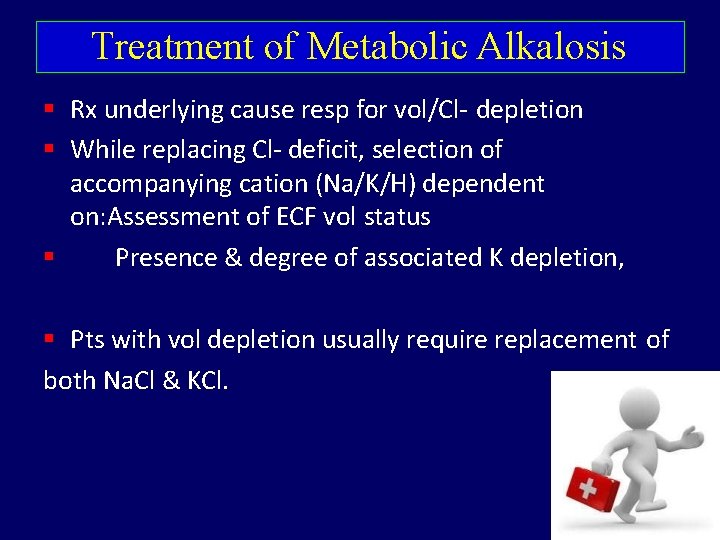
Treatment of Metabolic Alkalosis Rx underlying cause resp for vol/Cl- depletion While replacing Cl- deficit, selection of accompanying cation (Na/K/H) dependent on: Assessment of ECF vol status Presence & degree of associated K depletion, Pts with vol depletion usually require replacement of both Na. Cl & KCl.
Dialysis • In presence of renal failure or severe fluid overload state in CHF, dialysis +/- UF may be reqd to exchange HCO 3 for Cl & correct metabolic alkalosis. Adjunct Therapy • PPI can be admn to gastric acid production in cases of Cl-depletion met alkalosis resulting from loss of gastric H+/Cl- (e. g. pernicious vomiting, req for continual removal of gastric secretions.
MILK-ALKALI SYNDROME & OTHER HYPERCALCEMIC STATES • Cessation of alkali ingestion & Ca sources (often milk and calcium carbonate) • Treatment of underlying cause of hypercalcemia • Cl- and Vol repletion for commonly associated vomiting
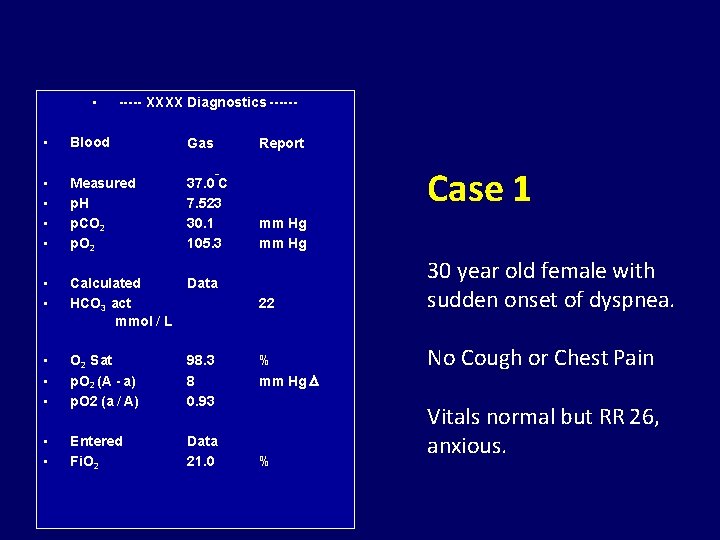
• ----- XXXX Diagnostics ------ • Blood Gas • • Measured p. H p. CO 2 p. O 2 37. 0 C 7. 523 30. 1 105. 3 • • Calculated HCO 3 act mmol / L Data • • • O 2 Sat p. O 2 (A - a) p. O 2 (a / A) 98. 3 8 0. 93 • • Entered Fi. O 2 Data 21. 0 Report Case 1 o mm Hg 22 % mm Hg % 30 year old female with sudden onset of dyspnea. No Cough or Chest Pain Vitals normal but RR 26, anxious.
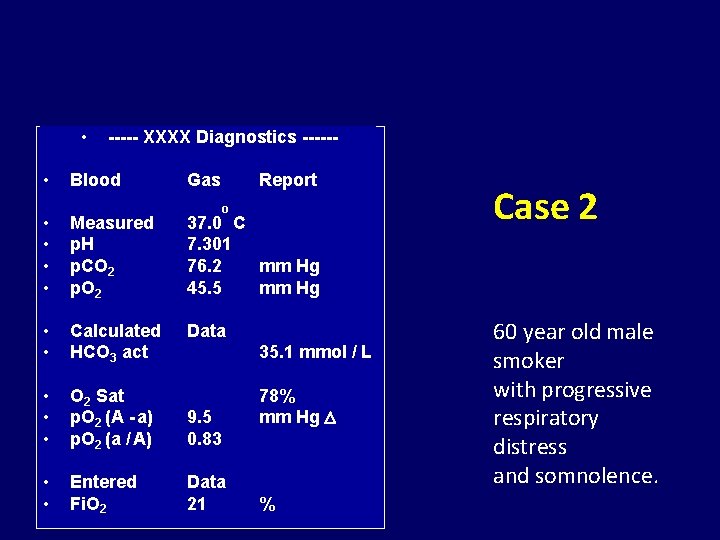
• • ----- XXXX Diagnostics ------ Blood Gas Report o • • Measured p. H p. CO 2 p. O 2 37. 0 C 7. 301 76. 2 mm Hg 45. 5 mm Hg • • Calculated HCO 3 act Data • • • O 2 Sat p. O 2 (A - a) p. O 2 (a / A) 9. 5 0. 83 • • Entered Fi. O 2 Data 21 35. 1 mmol / L 78% mm Hg % Case 2 60 year old male smoker with progressive respiratory distress and somnolence.
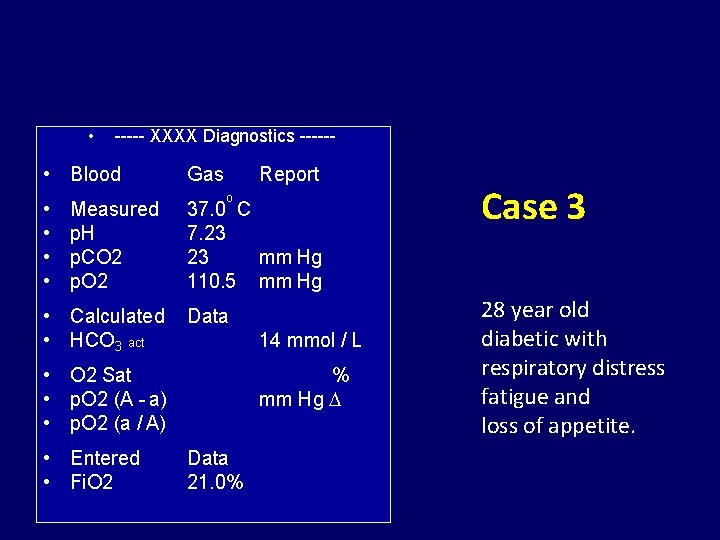
• ----- XXXX Diagnostics ------ • Blood • • Measured p. H p. CO 2 p. O 2 • Calculated • HCO 3 act Gas Report o 37. 0 C 7. 23 23 mm Hg 110. 5 mm Hg Data 14 mmol / L • O 2 Sat • p. O 2 (A - a) • p. O 2 (a / A) • Entered • Fi. O 2 % mm Hg Data 21. 0% Case 3 28 year old diabetic with respiratory distress fatigue and loss of appetite.



8) I shall practice gentle mechanical ventilation and not to try bring ABG to perfect normal. 9) I shall treat the patient, not the ABG report. 10) I shall always correlate ABG report clinically.

References ICU Book, The, 3 rd Edition - Paul L. Marino Diagnosing Acid-Base Disorders : JAPI • VOL. 54 • SEPTEMBER 2006 Harrison‘s PRINCIPLES OF INTERNAL MEDICINE Eighteenth Edition Washington Manual of Critical Care - 2 nd Ed Selected Websites – Listed in next slide

References • Selected Acid-Base Web Sites http: //www. acid-base. com/ http: //www. qldanaesthesia. com/Acid. Base. Book/ http: //www. virtual-anaesthesia-textbook. com /vat/acidbase. html#acidbase http: //ajrccm. atsjournals. org/cgi/content/full/162/6/2246 http: //www. osa. suite. dk/Osa. Textbook. htm http: //www. postgradmed. com/issues/2000/03_00/fall. htm http: //lungpowerpoints. com http: //uptodate. com


- Slides: 84