Acid base balance Dr S Parthasarathy MD DA

Acid base balance Dr. S. Parthasarathy MD. , DA. , DNB, MD (Acu), Dip. Diabetes Diploma in Software based statistics Ph. D ( physiology), IDRA , FICA Certifícate in USGRA

A lot of confusion !! • What is what ? • Sometimes anions are called bases ? • Sometimes called acids • E. g. bicarbonate , chloride • Know the definition !!
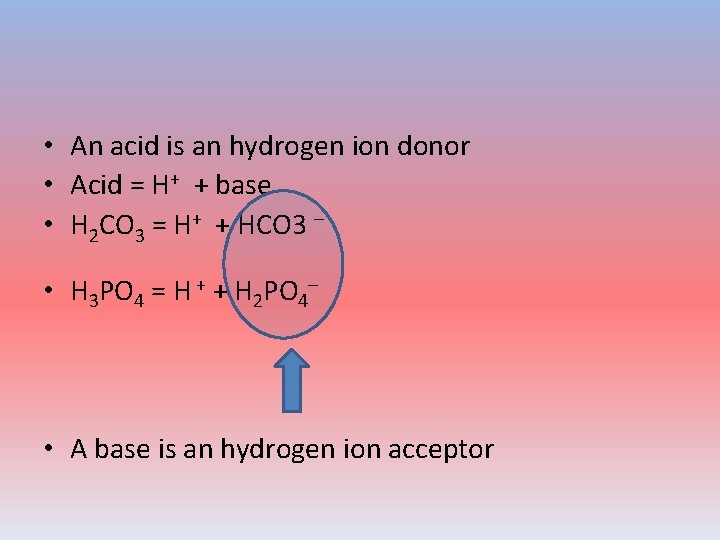
• An acid is an hydrogen ion donor • Acid = H+ + base • H 2 CO 3 = H+ + HCO 3 – • H 3 PO 4 = H + + H 2 PO 4– • A base is an hydrogen ion acceptor
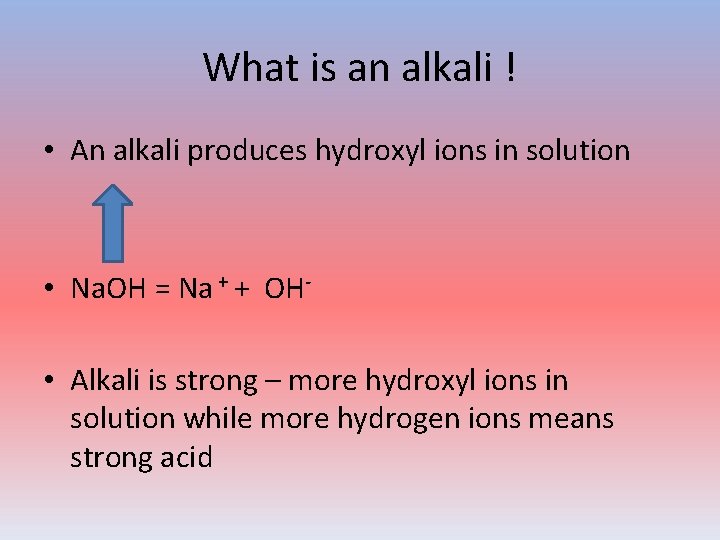
What is an alkali ! • An alkali produces hydroxyl ions in solution • Na. OH = Na + + OH • Alkali is strong – more hydroxyl ions in solution while more hydrogen ions means strong acid

Sorenson !! • In 1909 , he found out that enzyme activity changes with minute or minimal changes of hydrogen ion concentration • 0. 0001 mole pr liter of hydrogen ion 10 -4 moles per liter 0. 000001 = 10 -6 moles per liter Write like this – take the power and make it positive 4 and 6 Negative logarithm of hydrogen ion concentration in moles per liter

• p. H change and hydrogen ion change is never linear • Example: a solution with a p. H of 4 has 10 times the hydrogen ion concentration of a solution with a p. H of 5 and 100 times the hydrogen ion concentration of a solution with a p. H of 6
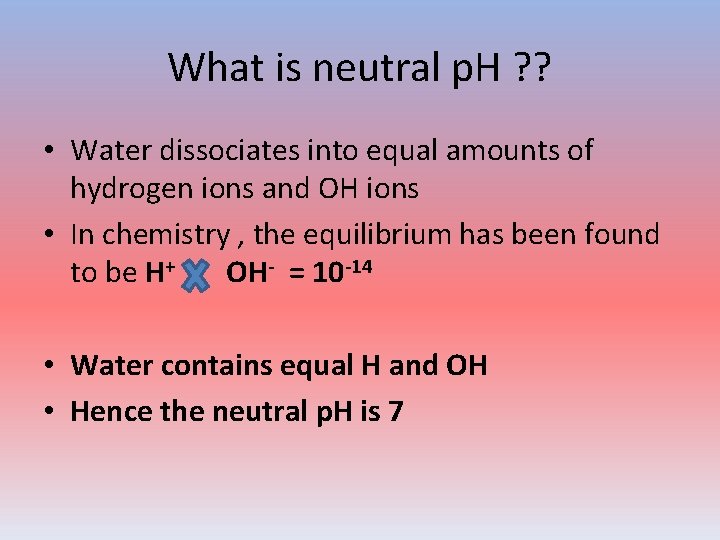
What is neutral p. H ? ? • Water dissociates into equal amounts of hydrogen ions and OH ions • In chemistry , the equilibrium has been found to be H+ OH- = 10 -14 • Water contains equal H and OH • Hence the neutral p. H is 7
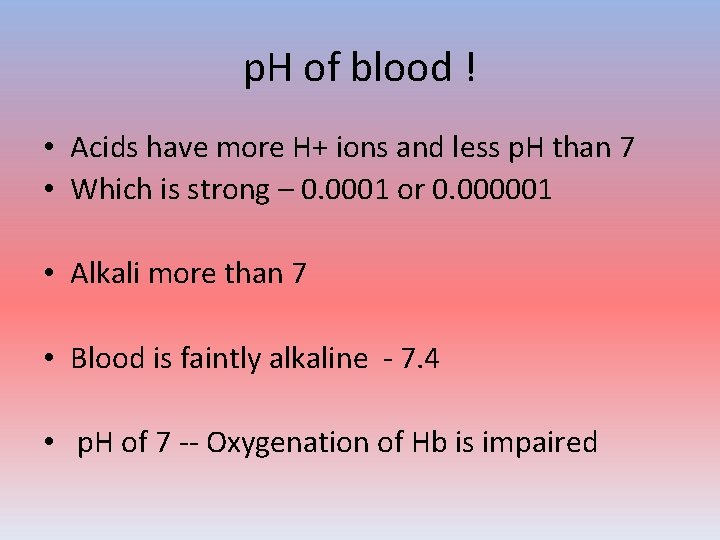
p. H of blood ! • Acids have more H+ ions and less p. H than 7 • Which is strong – 0. 0001 or 0. 000001 • Alkali more than 7 • Blood is faintly alkaline - 7. 4 • p. H of 7 -- Oxygenation of Hb is impaired
![Henderson hasselbach • p. H=p. Ka + log([k]/[HA]) • lactic acid (p. Ka, = Henderson hasselbach • p. H=p. Ka + log([k]/[HA]) • lactic acid (p. Ka, =](http://slidetodoc.com/presentation_image_h2/103f4fb9fdb83d2cf050cbcdaad64a91/image-9.jpg)
Henderson hasselbach • p. H=p. Ka + log([k]/[HA]) • lactic acid (p. Ka, = 3. 9) is a stronger acid than carbonic acid (p. Ka, = 6. 1) • because, at any given p. H, lactic acid will be more dissociated and therefore will release more H+ than carbonic acid.
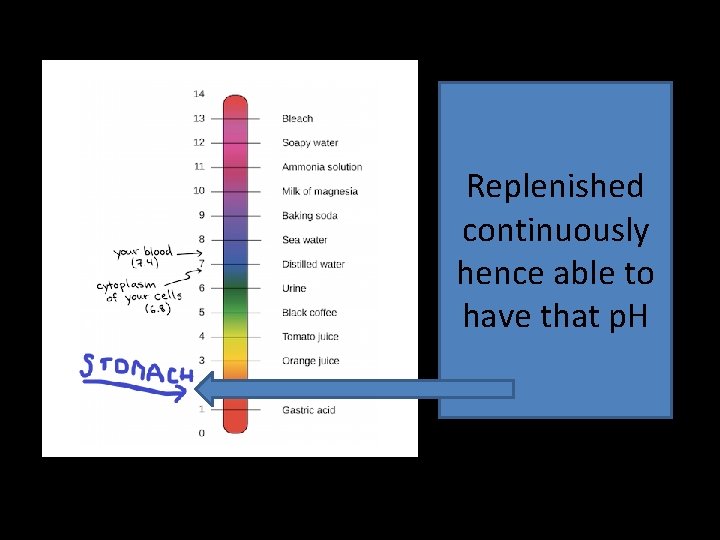
Replenished continuously hence able to have that p. H
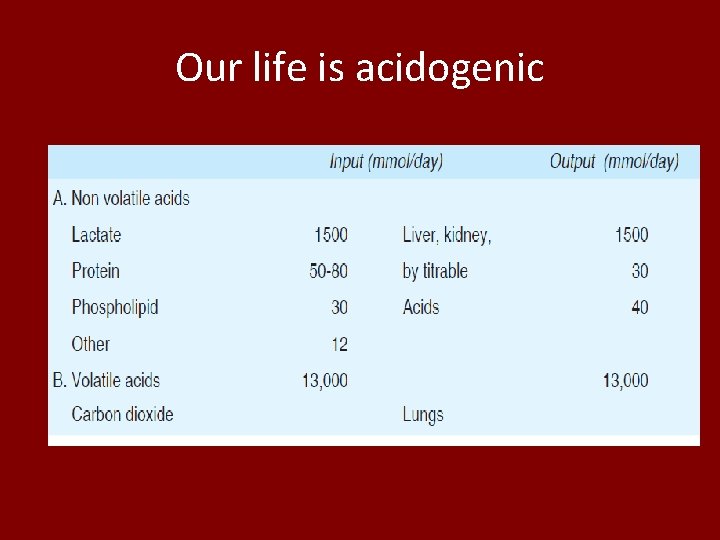
Our life is acidogenic

• Approx 10 neq for 0. 1 p. H change • We need to have a p. H between 7. 36 – 7. 44 approx • What happens if it changes • It is dangerous • then how it is avoided

Buffers !! • A buffer is a solution containing either a weak acid and its salt or a weak base and its salt, which is resistant to changes in p. H. • Fast p. H changes are not allowed
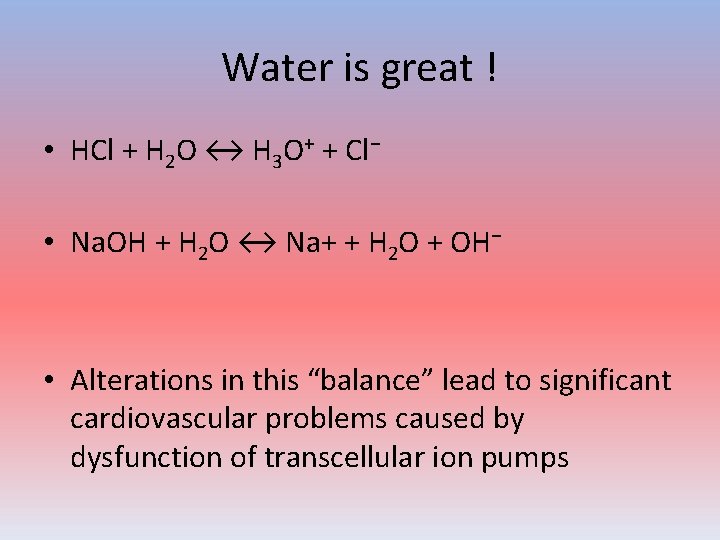
Water is great ! • HCl + H 2 O ↔ H 3 O+ + Cl− • Na. OH + H 2 O ↔ Na+ + H 2 O + OH− • Alterations in this “balance” lead to significant cardiovascular problems caused by dysfunction of transcellular ion pumps

ECF • The body's chemical buffer system consists of three individual buffers out of which the carbonic acid bicarbonate buffer is the most important. ( EC water ) • Phosphate buffer • Protein buffer
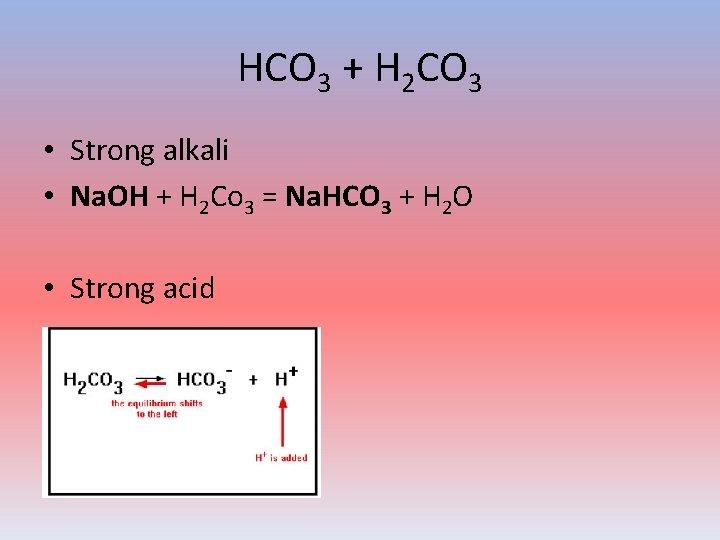
HCO 3 + H 2 CO 3 • Strong alkali • Na. OH + H 2 Co 3 = Na. HCO 3 + H 2 O • Strong acid
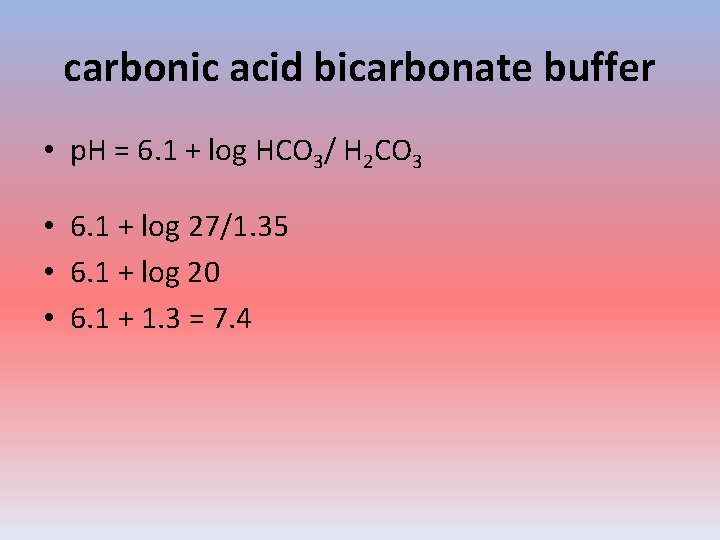
carbonic acid bicarbonate buffer • p. H = 6. 1 + log HCO 3/ H 2 CO 3 • 6. 1 + log 27/1. 35 • 6. 1 + log 20 • 6. 1 + 1. 3 = 7. 4
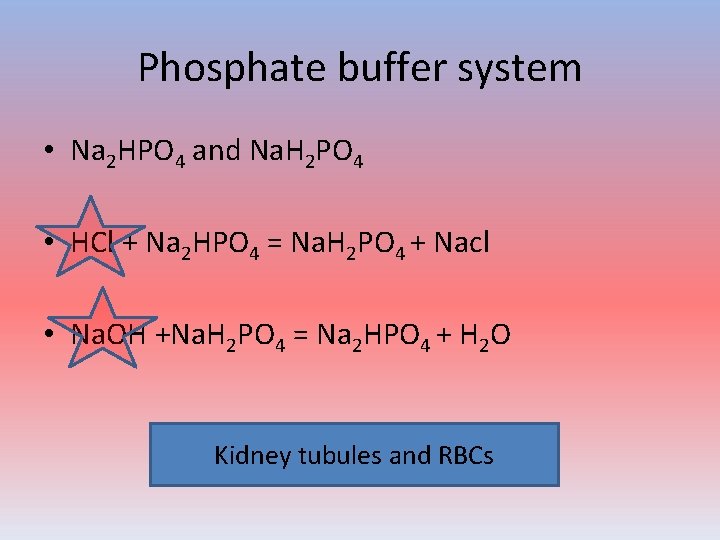
Phosphate buffer system • Na 2 HPO 4 and Na. H 2 PO 4 • HCl + Na 2 HPO 4 = Na. H 2 PO 4 + Nacl • Na. OH +Na. H 2 PO 4 = Na 2 HPO 4 + H 2 O Kidney tubules and RBCs
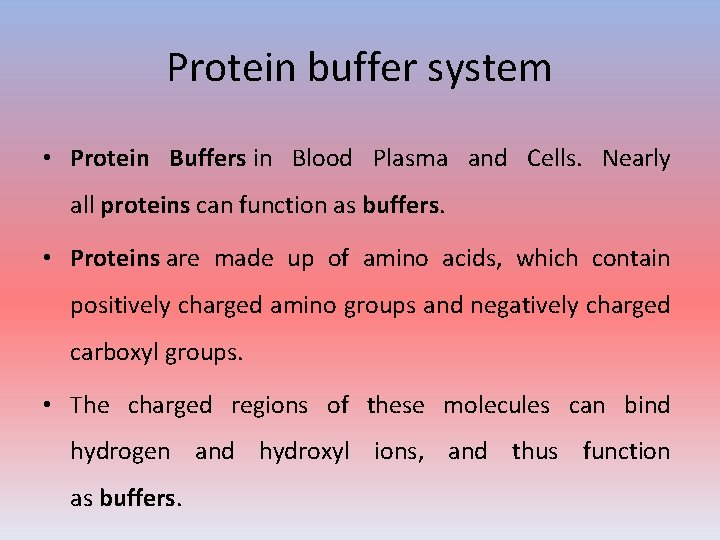
Protein buffer system • Protein Buffers in Blood Plasma and Cells. Nearly all proteins can function as buffers. • Proteins are made up of amino acids, which contain positively charged amino groups and negatively charged carboxyl groups. • The charged regions of these molecules can bind hydrogen and hydroxyl ions, and thus function as buffers.
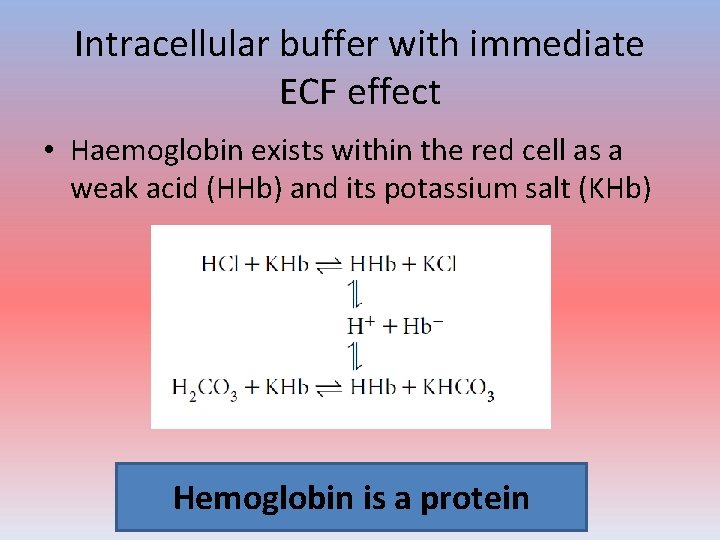
Intracellular buffer with immediate ECF effect • Haemoglobin exists within the red cell as a weak acid (HHb) and its potassium salt (KHb) Hemoglobin is a protein


Can we repeat ? ? Or go ahead ?
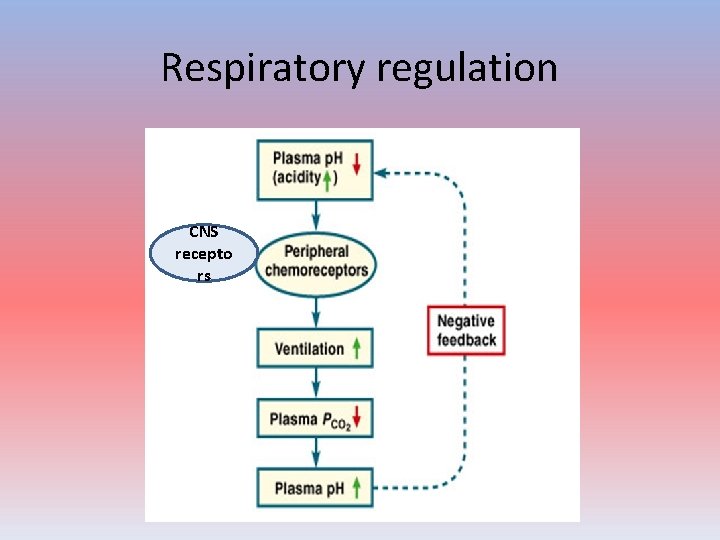
Respiratory regulation CNS recepto rs

RS - works how ? • Doubling the respiratory rate for less than 1 minute, removing “extra” CO 2, would increase the blood p. H by 0. 2. • This situation is common if you are exercising strenuously over a period of time. To keep up the necessary energy production, you would produce excess CO 2(and lactic acid if exercising beyond your aerobic threshold). • This helps to keep you from developing acidosis.
Physiology ! • Medullary CNS receptors • CO 2 more permeable than HCO 3 • Ventilation increase • Peripheral carotid receptors – more hyoxemic insult

• Upto 7 stimulation – hyperventilation • But below 7 – may not have the effect • Think of kussmaul respiration • Think of carbondioxide narcosis
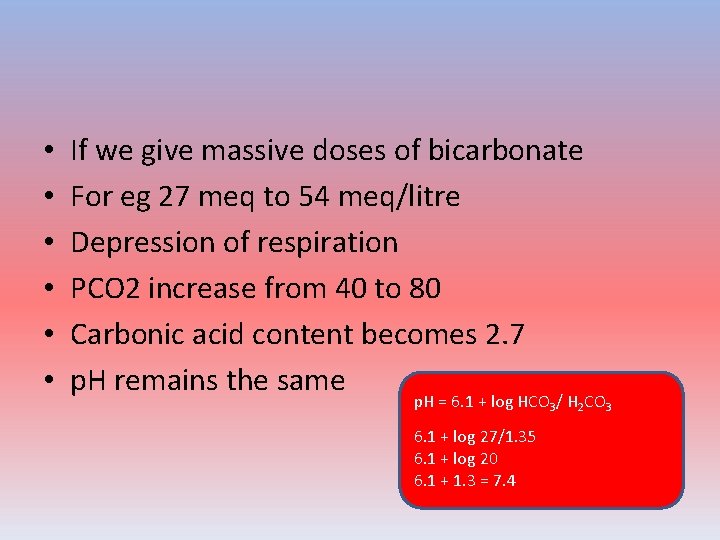
• • • If we give massive doses of bicarbonate For eg 27 meq to 54 meq/litre Depression of respiration PCO 2 increase from 40 to 80 Carbonic acid content becomes 2. 7 p. H remains the same p. H = 6. 1 + log HCO 3/ H 2 CO 3 6. 1 + log 27/1. 35 6. 1 + log 20 6. 1 + 1. 3 = 7. 4
Renal regulation • The kidneys control acid-base balance by excreting either acidic or basic urine • Excreting acidic urine reduces the amount of acid in extracellular fluid • Excreting basic urine removes base from the extracellular fluid
Three mechanisms • Reabsorption of bicarbonate • Acidification of buffer salts • Secretion of ammonia
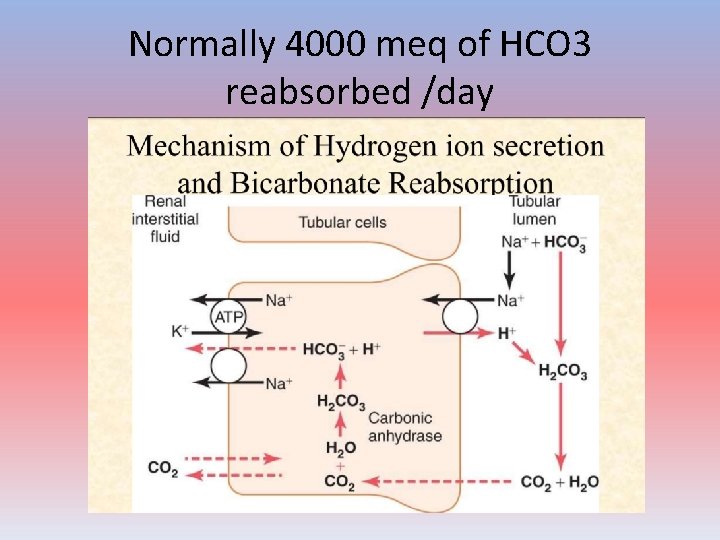
Normally 4000 meq of HCO 3 reabsorbed /day
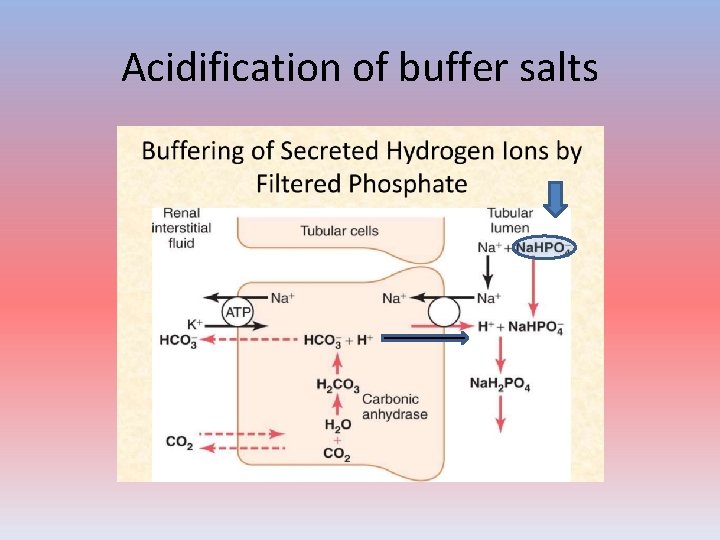
Acidification of buffer salts

Oxidation of glutamine

• Urine p. H may vary from 4. 5 to 7. 8 • What is normal ? ?
Inter-relation of buffers • HC 03 - is a useful marker of metabolic acid changes but not in a linear fashion. We expect the bicarb to decrease this much ? But does this happen ? Changes in HC 03 - are dependent upon respiratory acid-base variations.
• ECF mechanisms are instantaneous • Respiratory may take a few hours • Kidneys may take hours to days • But mechanisms by the kidneys permanent

• Thank you all
- Slides: 36