Acid Base Balance B 260 Fundamentals of Nursing

Acid Base Balance B 260 Fundamentals of Nursing

What is p. H? • p. H is the concentration of hydrogen (H+) ions • The p. H of blood indicates the net result of normal acid-base regulation, any acid-base imbalance, and the body’s compensatory mechanisms • The human body must maintain a very narrow p. H range • 7. 35 -7. 45

What is p. H? • In terms of the human body: • acidosis<------7. 35 -7. 45 ------>alkalosis • Carbon dioxide is the “acid” (CO 2) • Normal: 35 -45 mm. Hg • Bicarbonate is the “base” (HCO 3) • Normal: 22 -26 m. Eq/L
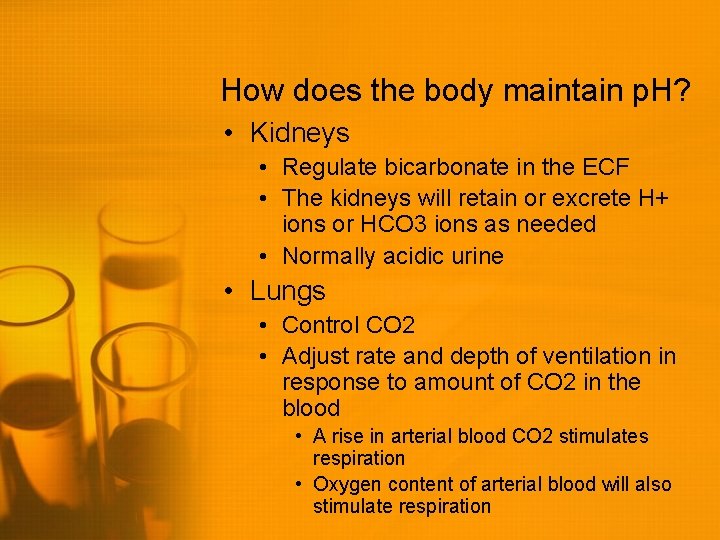
How does the body maintain p. H? • Kidneys • Regulate bicarbonate in the ECF • The kidneys will retain or excrete H+ ions or HCO 3 ions as needed • Normally acidic urine • Lungs • Control CO 2 • Adjust rate and depth of ventilation in response to amount of CO 2 in the blood • A rise in arterial blood CO 2 stimulates respiration • Oxygen content of arterial blood will also stimulate respiration

Acidosis and Alkalosis • Metabolic acidosis • Decreased HCO 3 or increase in other acids • Metabolic alkalosis • Increased HCO 3 and excess loss of acids • Respiratory acidosis • Increased Pa. CO 2 due to hypoventilation • Respiratory alkalosis • Decreased Pa. C 02 due to hyperventilation
Imbalances • Imbalances in Pa. CO 2 are influenced by respiratory causes • Imbalances in HCO 3 are influenced by metabolic processes
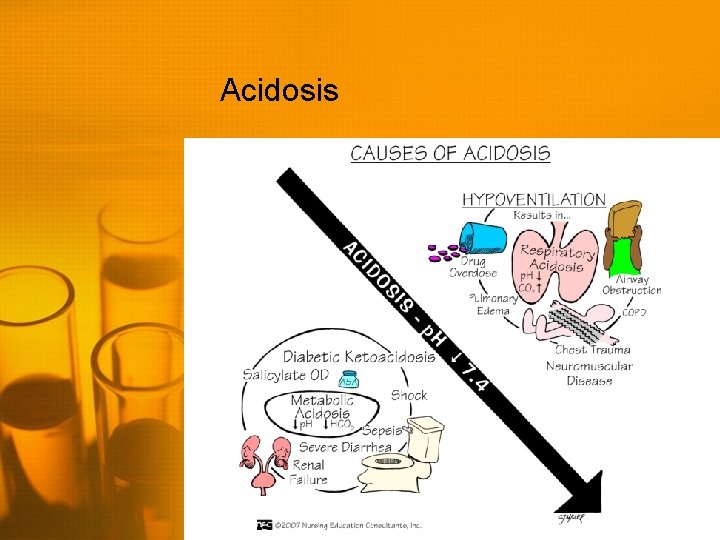
Acidosis
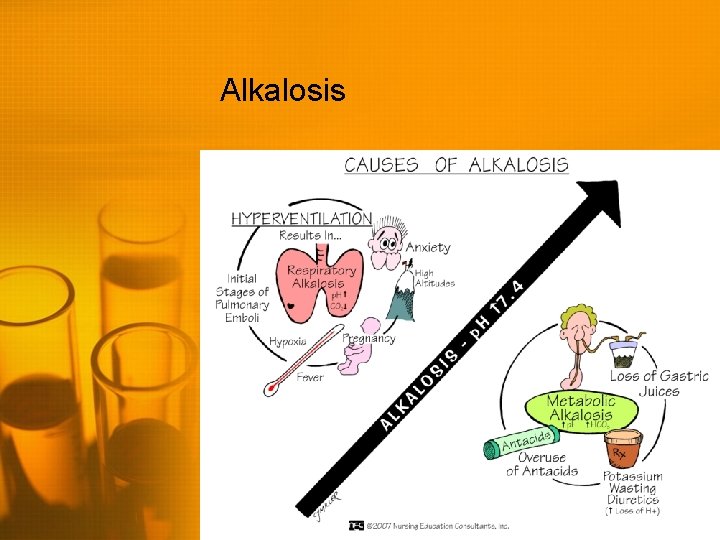
Alkalosis

Metabolic Acidosis • Low p. H (<7. 35) • Low HCO 3 (<22 m. Eq/L) Primary feature is decrease in serum HCO 3 • Body may attempt to compensate by increasing respirations to decrease CO 2 • Hyperkalemia may also occur due to shift of potassium out of the cells • Hypokalemia may occur once the acidosis is corrected • Treatment is aimed at correcting the metabolic defect • IV bicarbonate • Potassium management • Dialysis
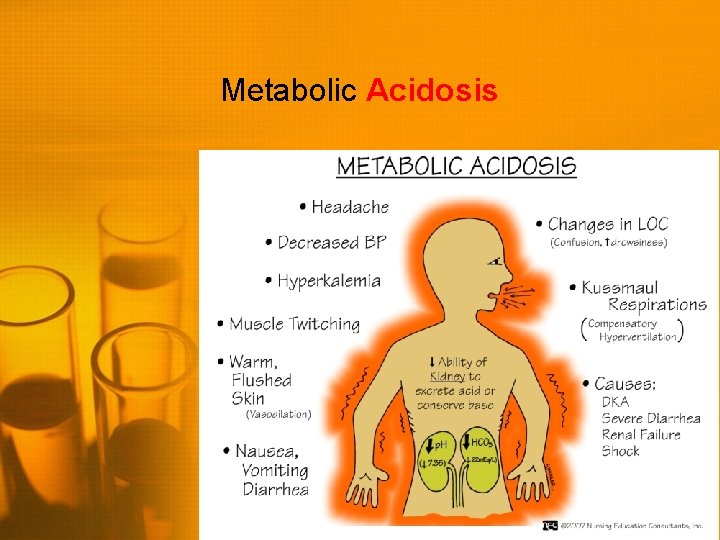
Metabolic Acidosis

Metabolic Alkalosis • High p. H (>7. 45) • High serum HCO 3 (>26) • Body may attempt to compensate by decreasing respirations to increase CO 2 • Treatment is aimed at treating the underlying disorder • • Chloride supplementation Restore normal fluid volume Maintain potassium Carbonic anhydrase inhibitor if unable to tolerate volume resuscitation
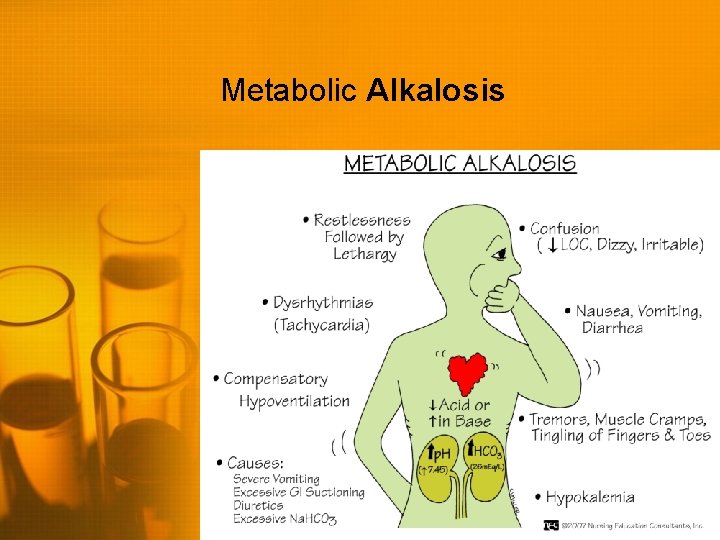
Metabolic Alkalosis
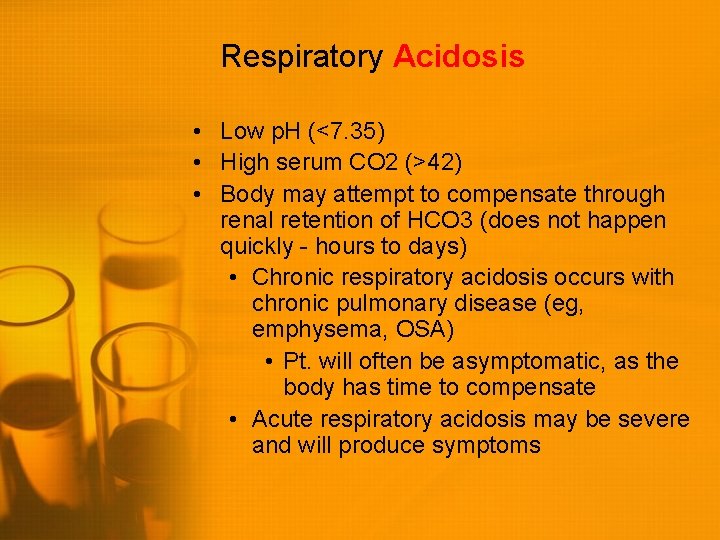
Respiratory Acidosis • Low p. H (<7. 35) • High serum CO 2 (>42) • Body may attempt to compensate through renal retention of HCO 3 (does not happen quickly - hours to days) • Chronic respiratory acidosis occurs with chronic pulmonary disease (eg, emphysema, OSA) • Pt. will often be asymptomatic, as the body has time to compensate • Acute respiratory acidosis may be severe and will produce symptoms

Respiratory Acidosis • Treatment is directed at improving ventilation --> treat the underlying cause • Pulmonary hygiene to clear respiratory tract • Adequate hydration to help clear secretions • Supplemental oxygen • Adjustment of mechanical ventilation as appropriate
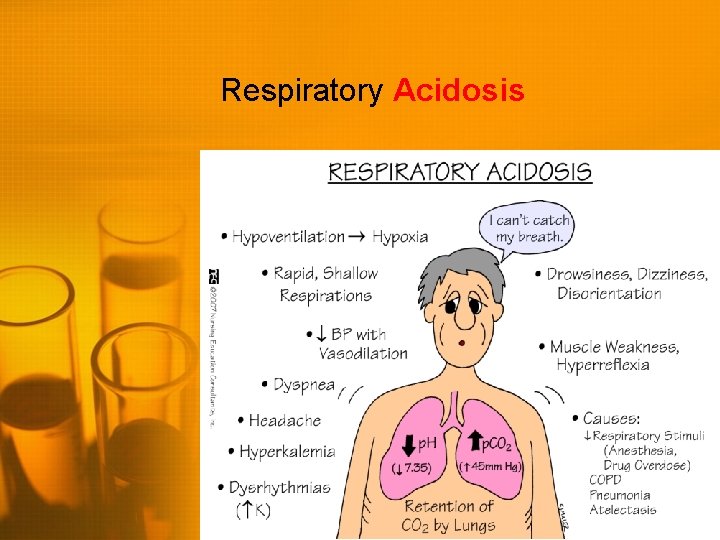
Respiratory Acidosis
Respiratory Alkalosis • • High p. H (>7. 45) Low Pa. CO 2 (<35) Always due to hyperventilation Body may compensate through increased kidney excretion of bicarbonate (does not happen quickly - hours to days) • Treatment is aimed at correcting the cause of hyperventilation • If anxiety-related, may breathe into a closed system (rebreathe CO 2)
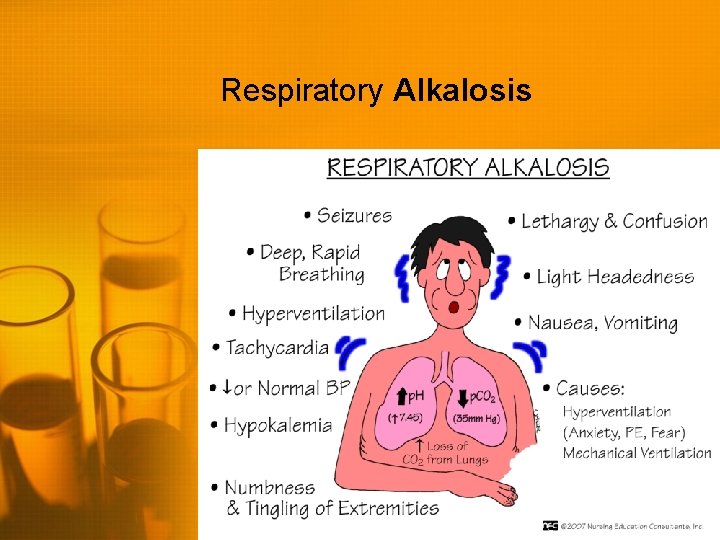
Respiratory Alkalosis
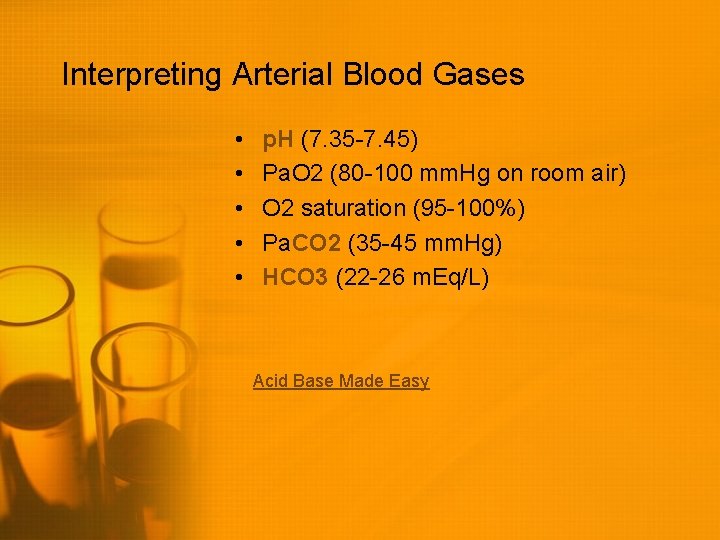
Interpreting Arterial Blood Gases • • • p. H (7. 35 -7. 45) Pa. O 2 (80 -100 mm. Hg on room air) O 2 saturation (95 -100%) Pa. CO 2 (35 -45 mm. Hg) HCO 3 (22 -26 m. Eq/L) Acid Base Made Easy
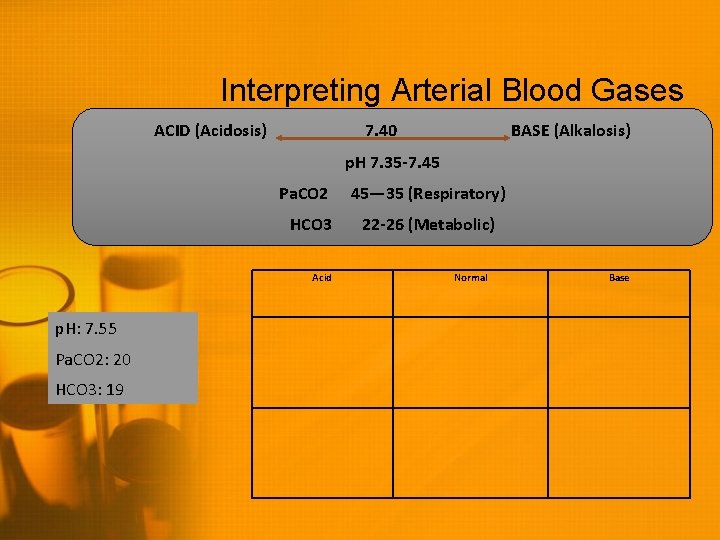
Interpreting Arterial Blood Gases ACID (Acidosis) 7. 40 BASE (Alkalosis) p. H 7. 35 -7. 45 Pa. CO 2 HCO 3 Acid p. H: 7. 55 Pa. CO 2: 20 HCO 3: 19 45— 35 (Respiratory) 22 -26 (Metabolic) Normal Base
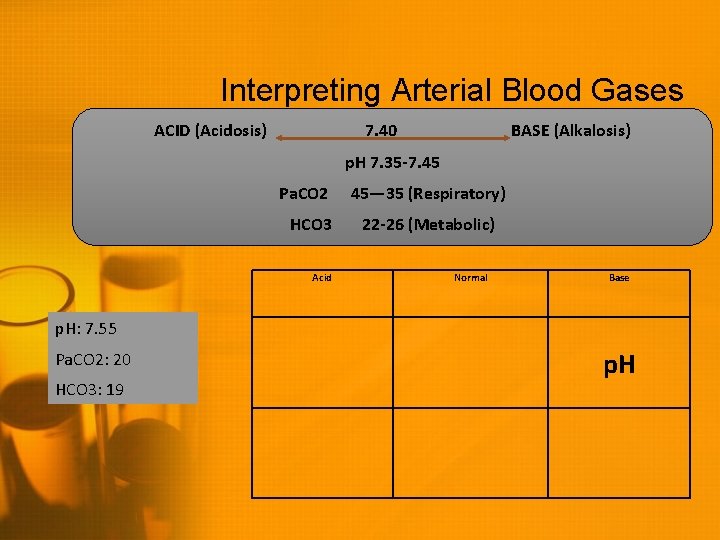
Interpreting Arterial Blood Gases ACID (Acidosis) 7. 40 BASE (Alkalosis) p. H 7. 35 -7. 45 Pa. CO 2 HCO 3 Acid 45— 35 (Respiratory) 22 -26 (Metabolic) Normal Base p. H: 7. 55 Pa. CO 2: 20 HCO 3: 19 p. H
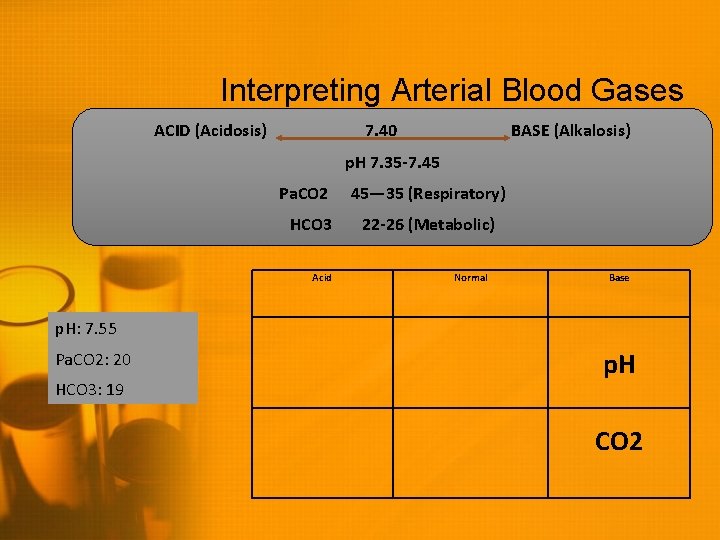
Interpreting Arterial Blood Gases ACID (Acidosis) 7. 40 BASE (Alkalosis) p. H 7. 35 -7. 45 Pa. CO 2 HCO 3 Acid 45— 35 (Respiratory) 22 -26 (Metabolic) Normal Base p. H: 7. 55 Pa. CO 2: 20 HCO 3: 19 p. H CO 2
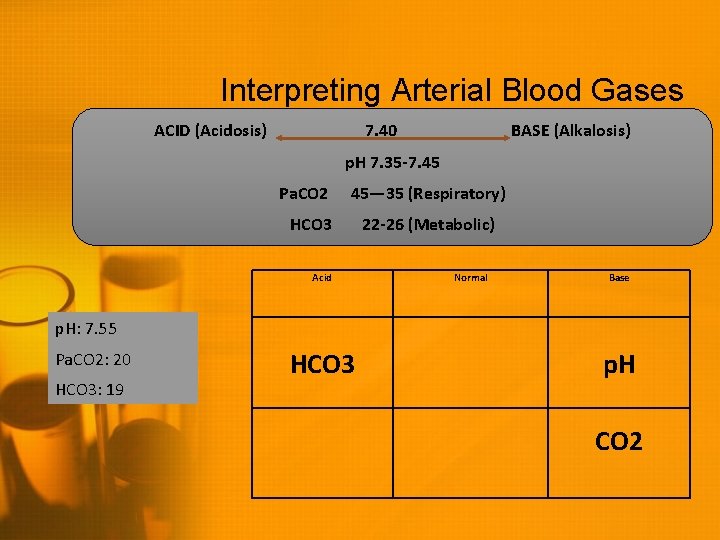
Interpreting Arterial Blood Gases ACID (Acidosis) 7. 40 BASE (Alkalosis) p. H 7. 35 -7. 45 Pa. CO 2 45— 35 (Respiratory) HCO 3 Acid 22 -26 (Metabolic) Normal Base p. H: 7. 55 Pa. CO 2: 20 HCO 3: 19 HCO 3 p. H CO 2
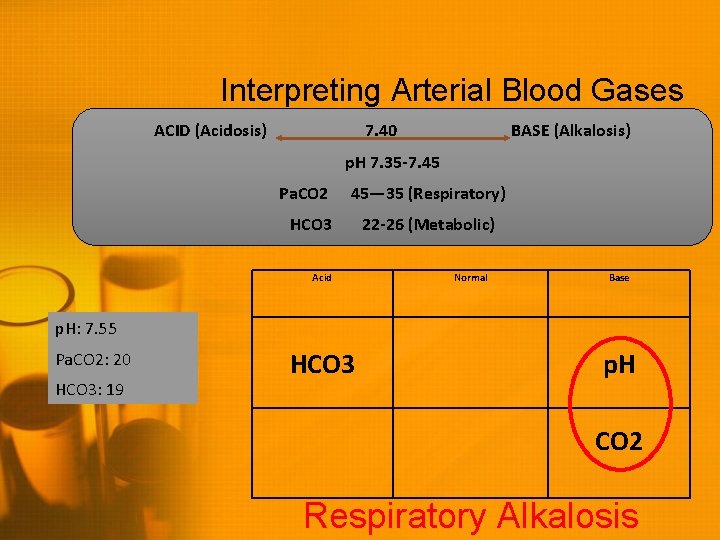
Interpreting Arterial Blood Gases ACID (Acidosis) 7. 40 BASE (Alkalosis) p. H 7. 35 -7. 45 Pa. CO 2 45— 35 (Respiratory) HCO 3 Acid 22 -26 (Metabolic) Normal Base p. H: 7. 55 Pa. CO 2: 20 HCO 3: 19 HCO 3 p. H CO 2 Respiratory Alkalosis
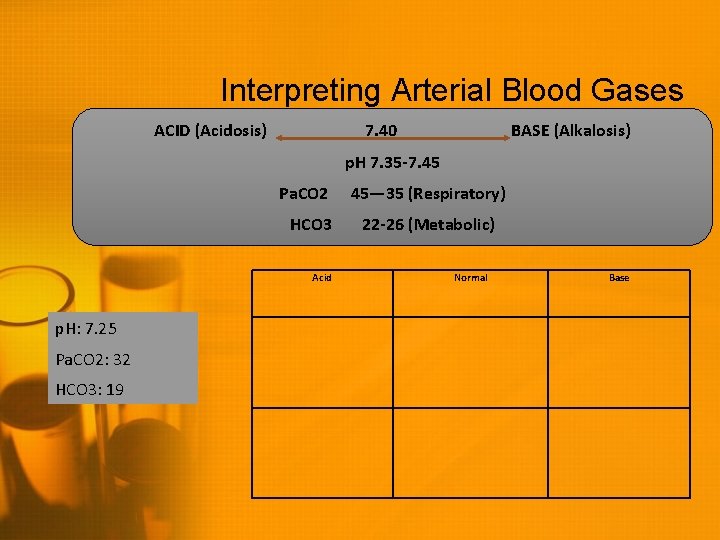
Interpreting Arterial Blood Gases ACID (Acidosis) 7. 40 BASE (Alkalosis) p. H 7. 35 -7. 45 Pa. CO 2 HCO 3 Acid p. H: 7. 25 Pa. CO 2: 32 HCO 3: 19 45— 35 (Respiratory) 22 -26 (Metabolic) Normal Base
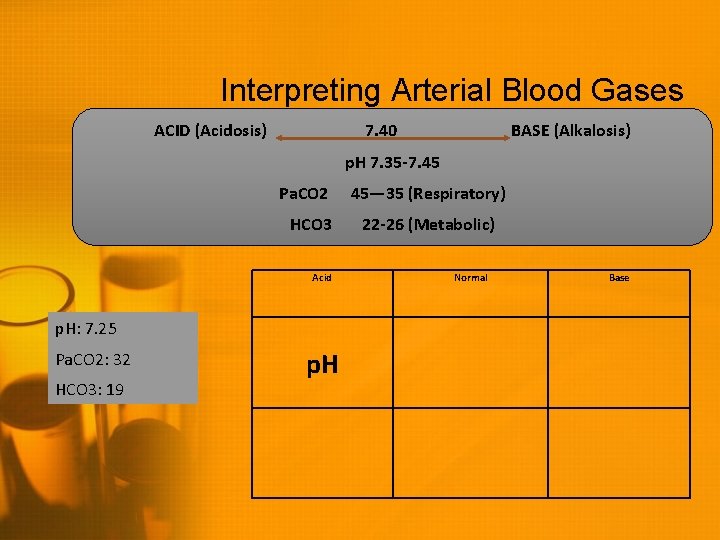
Interpreting Arterial Blood Gases ACID (Acidosis) 7. 40 BASE (Alkalosis) p. H 7. 35 -7. 45 Pa. CO 2 HCO 3 Acid p. H: 7. 25 Pa. CO 2: 32 HCO 3: 19 p. H 45— 35 (Respiratory) 22 -26 (Metabolic) Normal Base
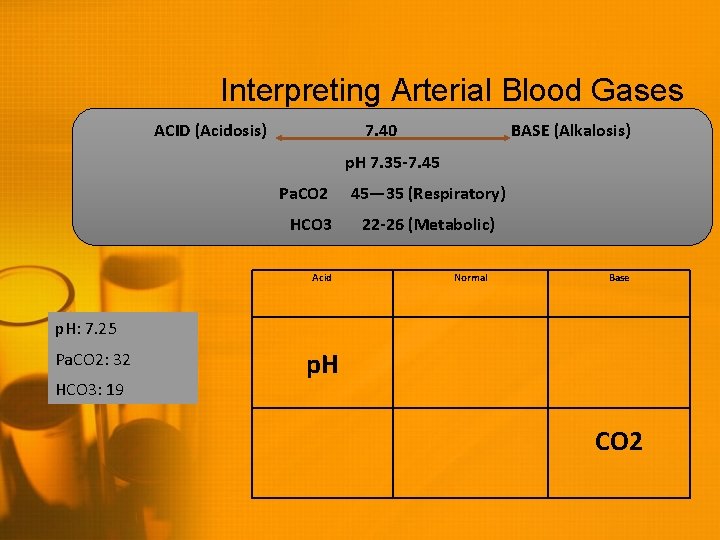
Interpreting Arterial Blood Gases ACID (Acidosis) 7. 40 BASE (Alkalosis) p. H 7. 35 -7. 45 Pa. CO 2 HCO 3 Acid 45— 35 (Respiratory) 22 -26 (Metabolic) Normal Base p. H: 7. 25 Pa. CO 2: 32 HCO 3: 19 p. H CO 2
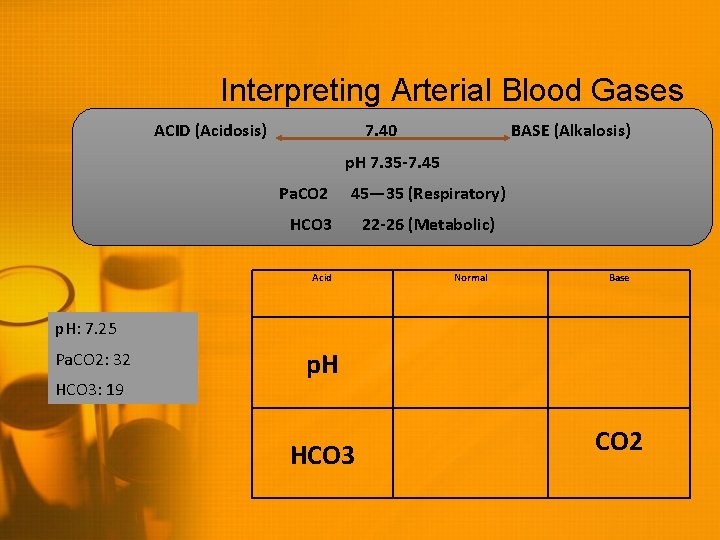
Interpreting Arterial Blood Gases ACID (Acidosis) 7. 40 BASE (Alkalosis) p. H 7. 35 -7. 45 Pa. CO 2 45— 35 (Respiratory) HCO 3 Acid 22 -26 (Metabolic) Normal Base p. H: 7. 25 Pa. CO 2: 32 HCO 3: 19 p. H HCO 3 CO 2
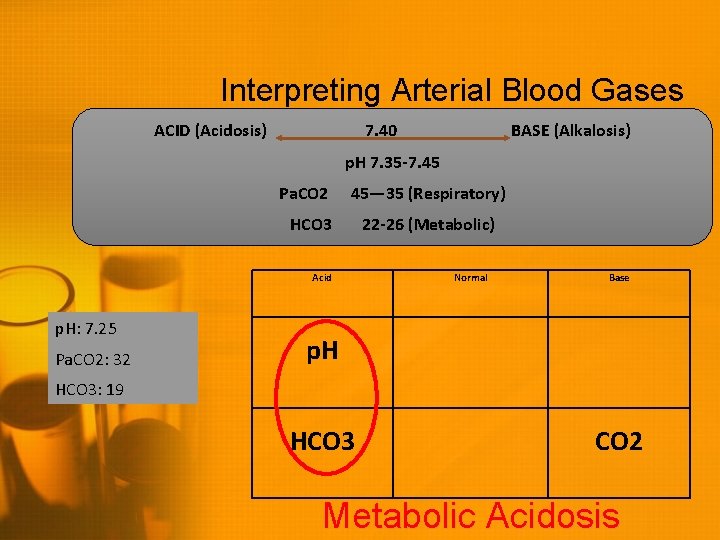
Interpreting Arterial Blood Gases ACID (Acidosis) 7. 40 BASE (Alkalosis) p. H 7. 35 -7. 45 Pa. CO 2 45— 35 (Respiratory) HCO 3 Acid p. H: 7. 25 Pa. CO 2: 32 22 -26 (Metabolic) Normal Base p. H HCO 3: 19 HCO 3 CO 2 Metabolic Acidosis

Practice p. H: 7. 25 Pa. CO 2: 55 HCO 3: 26 p. H: 7. 54 Pa. CO 2: 24 HCO 3: 25 p. H: 7. 55 Pa. CO 2: 20 HCO 3: 19 p. H: 7. 48 Pa. CO 2: 38 HCO 3: 28 p. H: 7. 4 Pa. CO 2: 42 HCO 3: 25 p. H: 7. 1 Pa. CO 2: 35 HCO 3: 18 p. H: 7. 15 Pa. CO 2: 46 HCO 3: 34 p. H: 7. 7 Pa. CO 2: 28 HCO 3: 23 p. H: 7. 5 Pa. CO 2: 48 HCO 3: 28 p. H: 7. 2 Pa. CO 2: 48 HCO 3: 24 p. H: 7. 17 Pa. CO 2: 35 HCO 3: 12 p. H: 7. 26 Pa. CO 2: 36 HCO 3: 16

Practice p. H: 7. 25 Pa. CO 2: 55 HCO 3: 26 p. H: 7. 55 Pa. CO 2: 20 HCO 3: 19 p. H: 7. 4 Pa. CO 2: 42 HCO 3: 25 p. H: 7. 15 Pa. CO 2: 46 HCO 3: 34 Resp. Acidosis p. H: 7. 54 Pa. CO 2: 24 Resp. Alkalosis HCO 3: 25 Resp. Alkalosis p. H: 7. 48 Pa. CO 2: 38 Met. Alkalosis HCO 3: 28 Normal p. H: 7. 1 Pa. CO 2: 35 Met. . Acidosis HCO 3: 18 Resp. Acidosis p. H: 7. 7 Pa. CO 2: 28 Resp. Alkalosis HCO 3: 23 p. H: 7. 5 p. H: 7. 2 Pa. CO 2: 48 Metabolic. Alkalosis Pa. CO 2: 48 Resp. Acidosis HCO 3: 28 HCO 3: 24 p. H: 7. 17 Pa. CO 2: 35 Metabolic. Acidosis HCO 3: 12 p. H: 7. 26 Pa. CO 2: 36 Met. . Acidosis HCO 3: 16
- Slides: 30