Acid Base Balance ABG Interpretation AcidBase Balance Maintain

Acid Base Balance ABG Interpretation
Acid-Base Balance Maintain a steady balance between acids and bases to achieve homeostasis Metabolic processes produce acids that must be neutralized and excreted Acid- donor of hydrogen Base- acceptor of hydrogen; HCO 3 is most abundant base in body fluids Buffer- substance that reacts with an acid or base to prevent large change in p. H Measure of H+ ion concentration Blood is slightly alkaline p. H 7. 35 to 7. 45
Regulators of Acid/Base Buffers: act chemically to neutralize acids /change strong acids to weak acids Primary regulators React immediately Cannot maintain p. H without adequate respiratory and renal function Example- Carbonic-acid bicarbonate: HCl + Na. HCO 3→Na. Cl + H 2 CO 3 Others- phosphates; proteins
Regulators of Acid/Base Respiratory system: eliminates CO 2 center (medulla) controls breathing Responds within minutes/hours to changes in acid/base ↑ respirations lead to ↑ CO 2 elimination and decreased CO 2 in blood p. CO 2 directly relates to carbonic acid concentration and p. H

Regulators of Acid/Base Under normal conditions, kidneys reabsorb all bicarbonate they filter Renal system can generate additional bicarbonate and eliminate excess hydrogen Secretion of hydrogen into renal tubule, combine hydrogen with NH 3, excretion of weak acids p. H of urine can range from 4 to 8 Responds within hours to days; can maintain balance indefinitely in chronic imbalances
Alterations in Acid-Base Balance Imbalance produced when 1: 20 ratio between acid and base content is altered Occurs when compensatory mechanisms fail (either overwhelmed or not enough time) Classification of imbalances Respiratory: affect carbonic acid concentration Metabolic: affect bicarbonate
Blood Gas Values Arterial blood gas (ABG) values provide information about Acid-base status Underlying cause of imbalance Body’s ability to regulate p. H Overall oxygen status
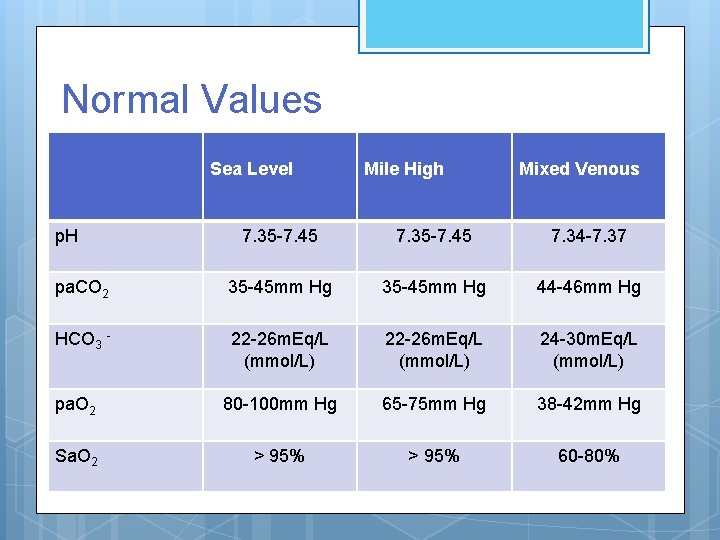
Normal Values Sea Level p. H Mile High Mixed Venous 7. 35 -7. 45 7. 34 -7. 37 pa. CO 2 35 -45 mm Hg 44 -46 mm Hg HCO 3 - 22 -26 m. Eq/L (mmol/L) 24 -30 m. Eq/L (mmol/L) pa. O 2 80 -100 mm Hg 65 -75 mm Hg 38 -42 mm Hg Sa. O 2 > 95% 60 -80%

Respiratory Acidosis p. H < 7. 35; Pa. CO 2 > 45 (hypercapnia) Carbonic acid excess caused by Hypoventilation Respiratory failure Compensation Kidneys urine conserve HCO 3 - and secrete H+ into

Resp. Acidosis. Behaviors/Treatment LOC, confusion, restlessness, stupor, coma Headache Tachycardia, dysrhythmias Warm/flushed skin Hyperkalemia Treatment- improve ventilation

Respiratory Alkalosis p. H > 7. 45; Pa. CO 2 < 45 Hyperventilation Primary cause is hypoxemia from acute pulmonary disorders Other causes- anxiety, fear, pain, head injury, fever, over-ventilation by MV Compensation- buffering with Cl- shift out of cell (acute); renal excretion of HCO 3 (chronic)

Respiratory Alkalosis. Behavior/Treatment Light-headedness, syncope, convulsions Weakness, paresthesia Hypocalcemia/hypokalemia Dysrhythmias Treatment- treat cause

Metabolic Acidosis p. H < 7. 35; HCO 3 - < 22 Gain an acid or lose a base Gain acid- DKA, uremia, acidic drugs, lactic acidosis, renal failure Lose base- diarrhea, pancreatic problems, laxatives Compensation- Kussmaul respiration; kidneys attempt to excrete additional acid

Metabolic Acidosis. Behavior/Treatment Behaviors of causative condition LOC, confusion, stupor, coma Kussmaul’s respirations Warm, flushed skin Hyperkalemia Treatment- treat cause, +/- bicarbonate

Metabolic Alkalosis p. H > 7. 45; HCO 3 - > 26 Gain a base or lose an acid Gain base- antacids, massive blood transfusions Lose acid- NGT suction, vomiting, steroids, diuretic therapy, hypokalemia Compensation- decreased respiratory rate; renal excretion of HCO 3 -

Metabolic Alkalosis. Behavior/Treatment CNS excitability, hyperactive reflexes, convulsions Dysrhythmias Hypokalemia Treatment- treat cause, K replacement, +/- Diamox

Mixed Acid-Base Disorders Two or more disorders present at the same time p. H depends on type, severity, and acuity of each of the disorders & compensatory mechanisms engaged Examples Cardiopulmonary arrest: hypoventilation & anaerobic metabolism Post-op pain & NGT suctioning
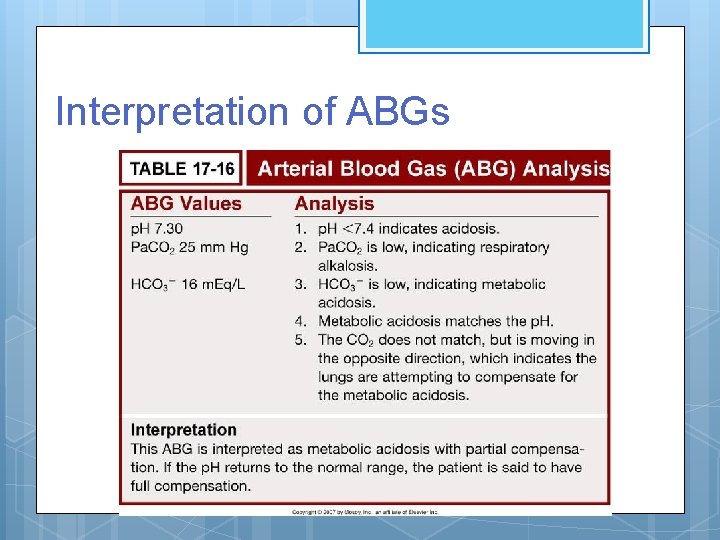
Interpretation of ABGs
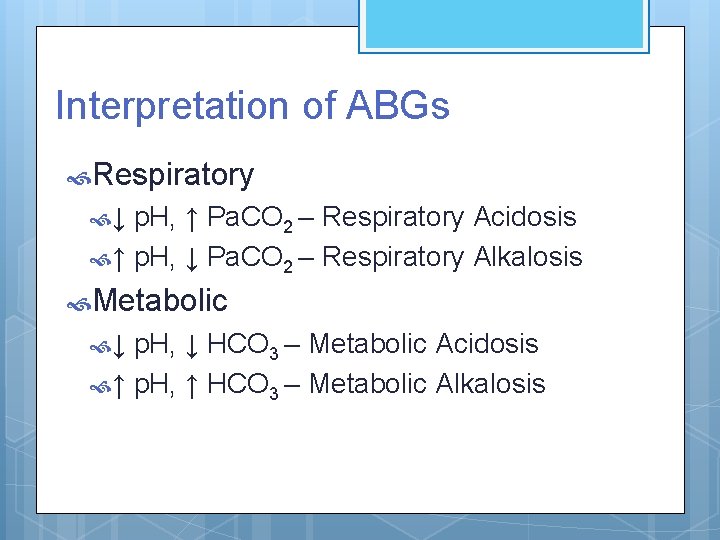
Interpretation of ABGs Respiratory ↓ p. H, ↑ Pa. CO 2 – Respiratory Acidosis ↑ p. H, ↓ Pa. CO 2 – Respiratory Alkalosis Metabolic ↓ p. H, ↓ HCO 3 – Metabolic Acidosis ↑ p. H, ↑ HCO 3 – Metabolic Alkalosis
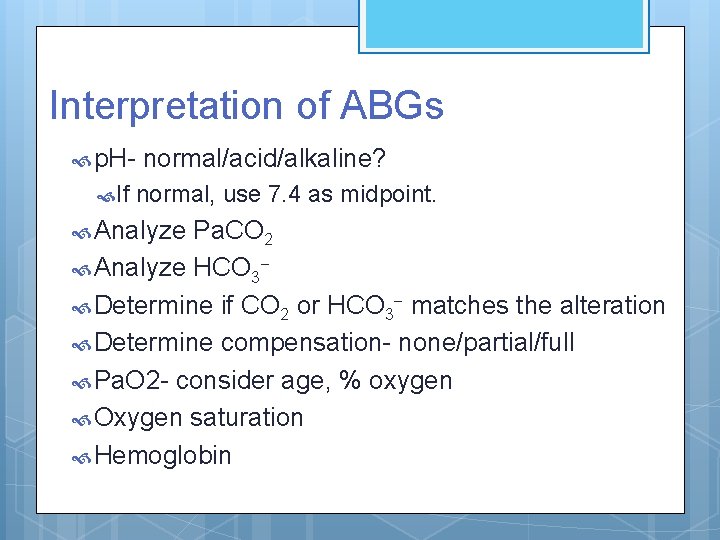
Interpretation of ABGs p. H If normal/acid/alkaline? normal, use 7. 4 as midpoint. Analyze Pa. CO 2 Analyze HCO 3 Determine if CO 2 or HCO 3 - matches the alteration Determine compensation- none/partial/full Pa. O 2 - consider age, % oxygen Oxygen saturation Hemoglobin
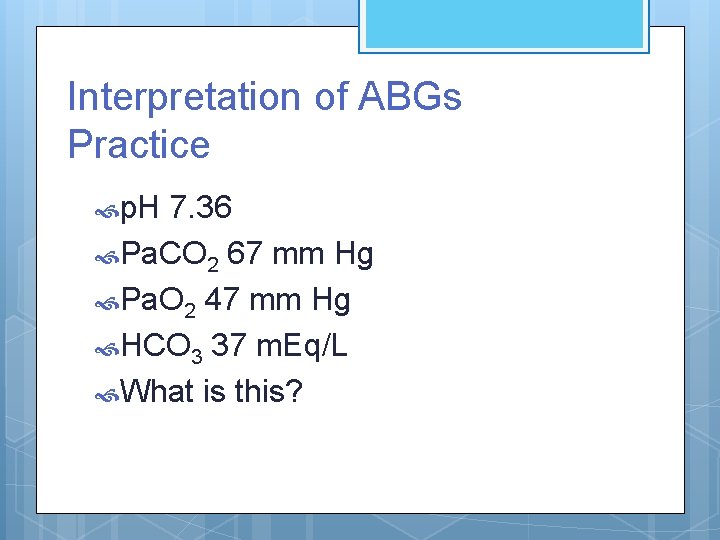
Interpretation of ABGs Practice p. H 7. 36 Pa. CO 2 67 mm Hg Pa. O 2 47 mm Hg HCO 3 37 m. Eq/L What is this?
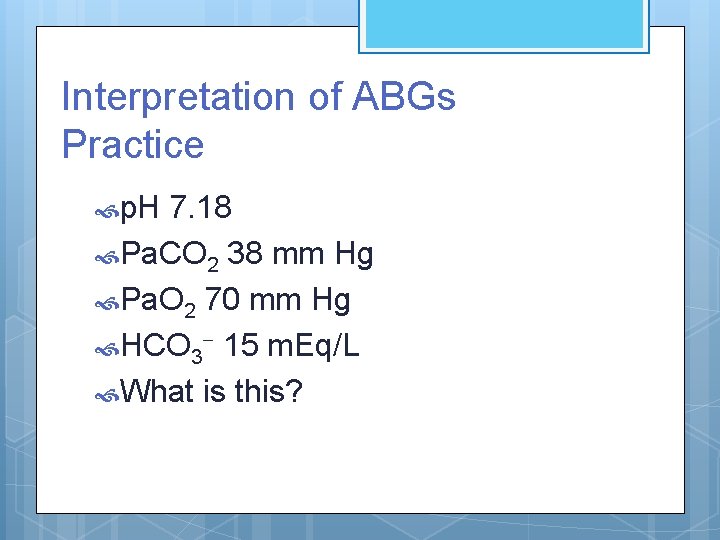
Interpretation of ABGs Practice p. H 7. 18 Pa. CO 2 38 mm Hg Pa. O 2 70 mm Hg HCO 3 - 15 m. Eq/L What is this?
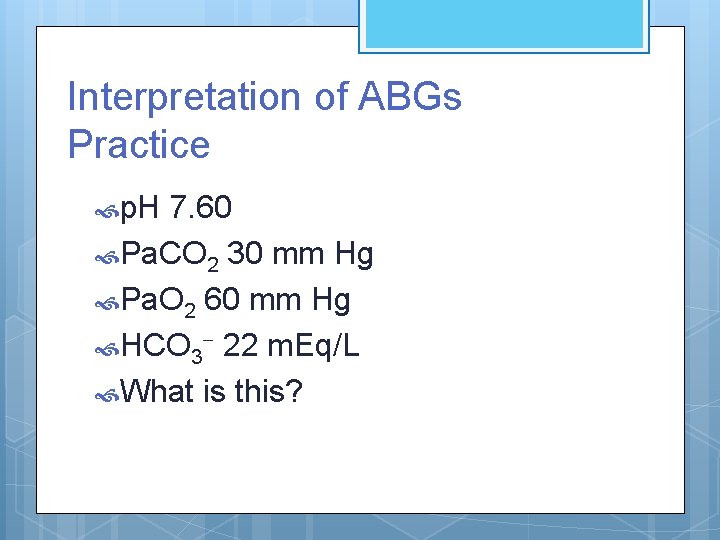
Interpretation of ABGs Practice p. H 7. 60 Pa. CO 2 30 mm Hg Pa. O 2 60 mm Hg HCO 3 - 22 m. Eq/L What is this?
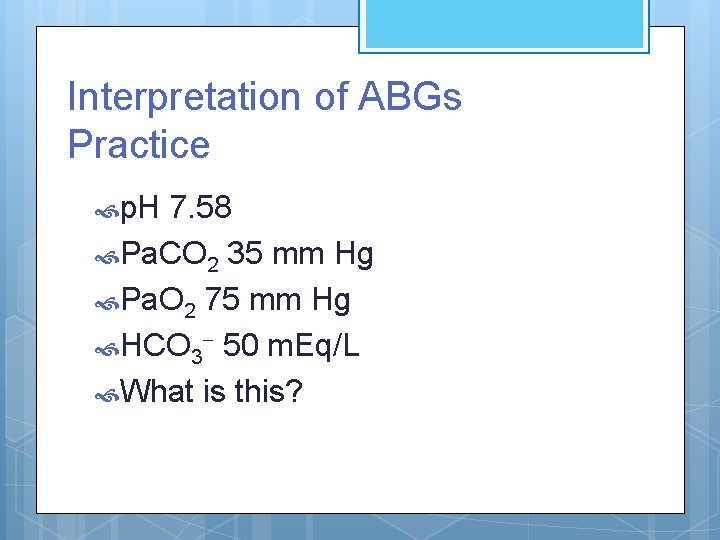
Interpretation of ABGs Practice p. H 7. 58 Pa. CO 2 35 mm Hg Pa. O 2 75 mm Hg HCO 3 - 50 m. Eq/L What is this?
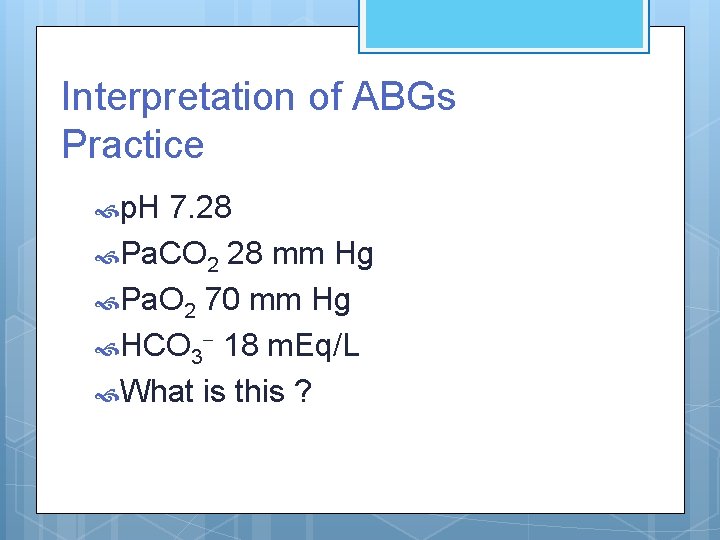
Interpretation of ABGs Practice p. H 7. 28 Pa. CO 2 28 mm Hg Pa. O 2 70 mm Hg HCO 3 - 18 m. Eq/L What is this ?

Case Study – Jeri’s been on a 3 -day party binge Friends are unable to awaken her Assessment reveals level of consciousness difficult to arouse Respiratory rate 8 Shallow breathing pattern Diminished breath sounds 1. 2. What ABGs do you expect? What is your treatment?

Case Study – Mayna Presented to the ER after a sexual assault Physical examination reveals hysterical emotional distress Respiratory rate 38 Lungs clear O 2 sat 96% 1. 2. What ABGs do you expect? What is your treatment?

Case Study – Glen History of fever, aches, and chills Generally feeling ill Cough productive of yellow, thick sputum for the past 4 days Examination reveals temp 38. 4° C Respiratory rate 20 Lungs with crackles in left lower lobes 1. 2. What ABGs do you expect? What is your treatment?

Case Study – Alan 17 years old with a history of Feeling bad Fatigue Constant thirst Frequent urination Blood sugar is 484 mg/dl Respirations are 28 and deep Breath has a fruity odor His lungs are clear 1. 2. What ABGs do you expect? What is your treatment?

Case Study – Anthony History of nausea and vomiting for the past week Has been self-medicating himself with baking soda to control his abdominal discomfort 1. 2. What ABGs do you expect? What is your treatment?

Case Study – Susan ABG results are: 1. 2. p. H 7. 20 Pa. CO 2 58 mm Hg Pa. O 2 59 mm Hg HCO 3 - 24 m. Eq/L Describe a patient who would have these ABGs, including history and assessment. What is the treatment?

Case Study – Fernando ABG results are: 1. 2. p. H 7. 39 Pa. CO 2 38 mm Hg Pa. O 2 44 mm Hg HCO 3 - 24 m. Eq/L Describe a patient who would have these ABGs, including history and assessment. What is the treatment?

Case Study – Brianna ABG results are: 1. 2. p. H 7. 36 Pa. CO 2 58 mm Hg Pa. O 2 50 mm Hg HCO 3 - 33 m. Eq/L Describe a patient who would have these ABGs, including history and assessment. What is the treatment?

Case Study – Monica ABG results are: 1. 2. p. H 7. 50 Pa. CO 2 28 mm Hg Pa. O 2 85 mm Hg HCO 3 - 24 m. Eq/L Describe a patient who would have these ABGs, including history and assessment. What is the treatment?

Case Study – Mike ABG results are: 1. 2. p. H 7. 20 Pa. CO 2 28 mm Hg Pa. O 2 81 mm Hg HCO 3 - 18 m. Eq/L Describe a patient who would have these ABGs, including history and assessment. What is the treatment?

Case Study – Jeremy ABG results are: 1. 2. p. H 7. 57 Pa. CO 2 46 mm Hg Pa. O 2 87 mm Hg HCO 3 - 38 m. Eq/L Describe a patient who would have these ABGs, including history and assessment. What is the treatment?
- Slides: 36