Acid and Bases Types of Oxides Many acids

Acid and Bases -Types of Oxides. Many acids and alkali are formed by dissolving oxides in water

At the end of this e-learning, you should able to: l Classify oxides as acidic, basic, amphoteric or neutral based on metallic/non-metallic character

What is an oxide? l An oxide is a compound of oxygen and another element. l Most oxides can be grouped into four types: acidic oxides l basic oxides l amphoteric oxides l neutral oxides l
Acidic oxides l Oxides of non-metal l Acidic oxides are often gases at room temperature.
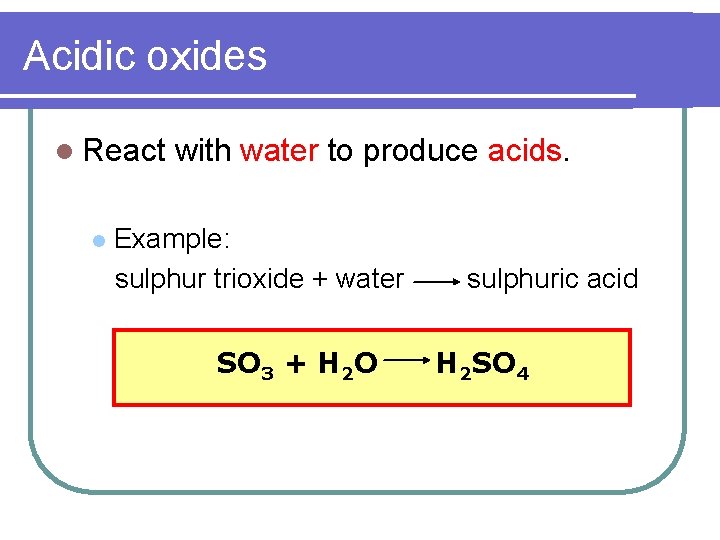
Acidic oxides l React l with water to produce acids. Example: sulphur trioxide + water SO 3 + H 2 O sulphuric acid H 2 SO 4
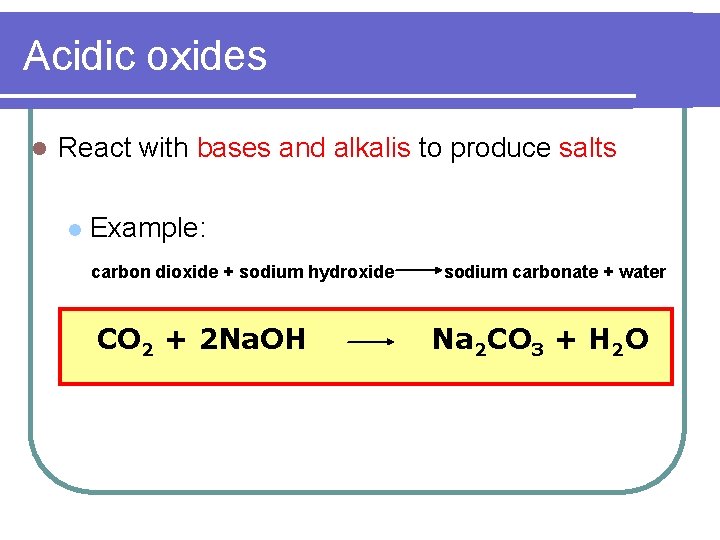
Acidic oxides l React with bases and alkalis to produce salts l Example: carbon dioxide + sodium hydroxide CO 2 + 2 Na. OH sodium carbonate + water Na 2 CO 3 + H 2 O
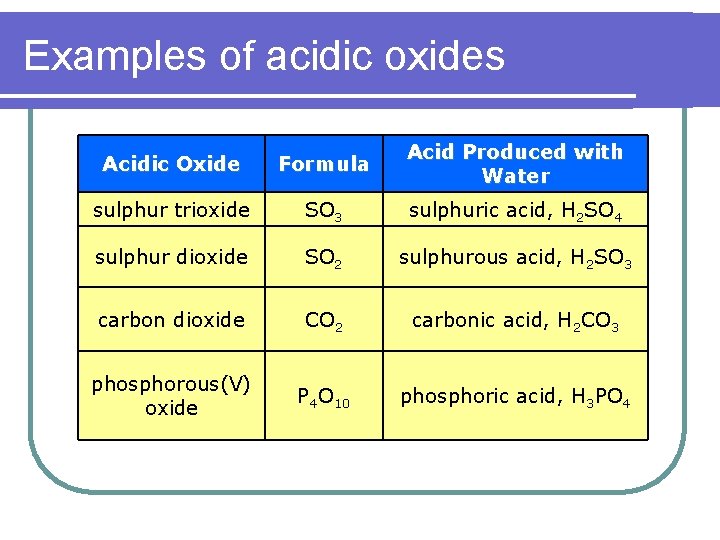
Examples of acidic oxides Acidic Oxide Formula Acid Produced with Water sulphur trioxide SO 3 sulphuric acid, H 2 SO 4 sulphur dioxide SO 2 sulphurous acid, H 2 SO 3 carbon dioxide CO 2 carbonic acid, H 2 CO 3 phosphorous(V) oxide P 4 O 10 phosphoric acid, H 3 PO 4

Basic oxides Oxides of metal l Basic oxides are often solids at room temperature. l Most basic oxides are insoluble in water. l Calcium oxide (quicklime)
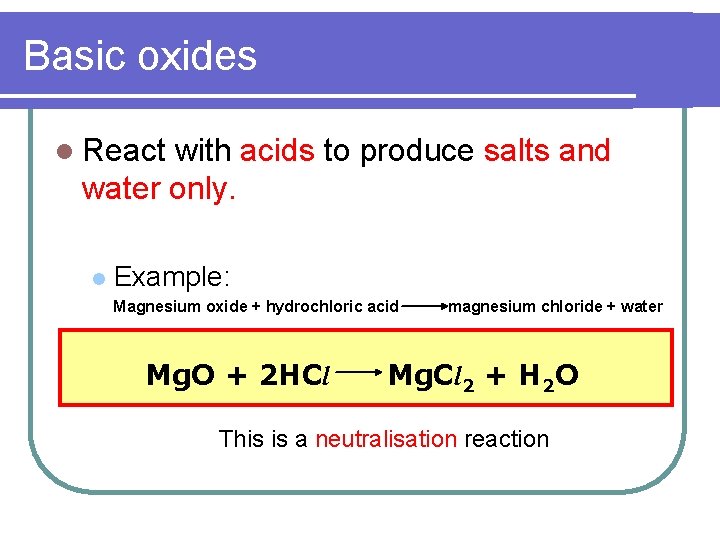
Basic oxides l React with acids to produce salts and water only. l Example: Magnesium oxide + hydrochloric acid Mg. O + 2 HCl magnesium chloride + water Mg. Cl 2 + H 2 O This is a neutralisation reaction
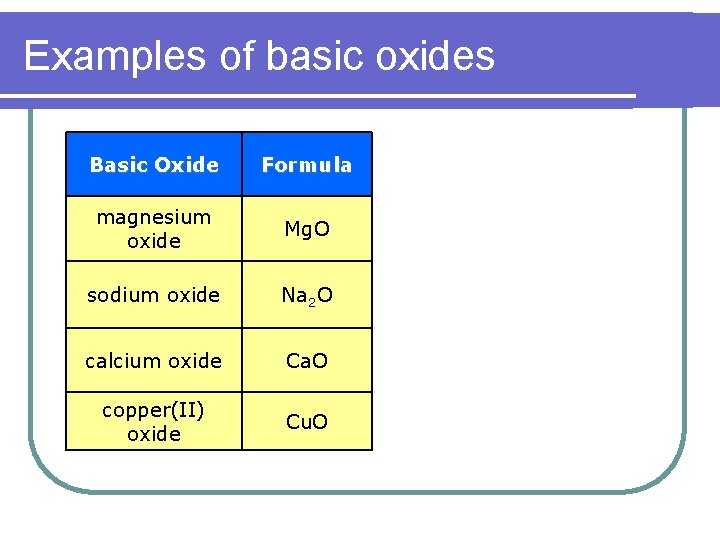
Examples of basic oxides Basic Oxide Formula magnesium oxide Mg. O sodium oxide Na 2 O calcium oxide Ca. O copper(II) oxide Cu. O

Amphoteric oxides Oxides of metal l Can behave as acidic oxides or as basic oxides l Zinc oxide
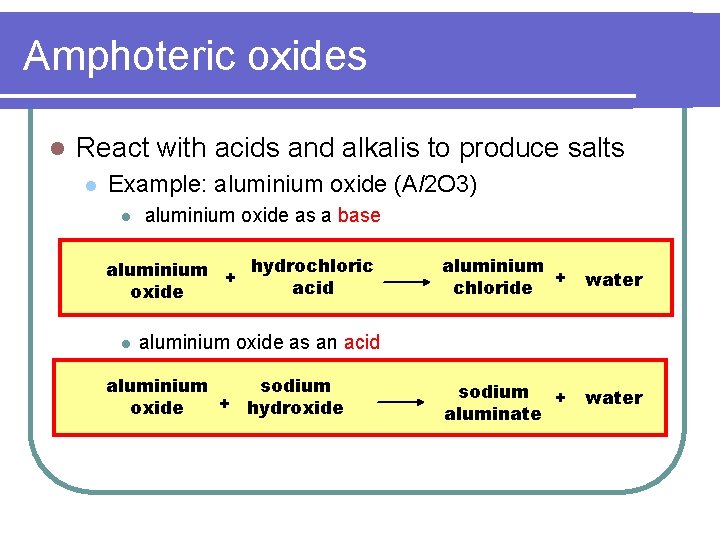
Amphoteric oxides l React with acids and alkalis to produce salts l Example: aluminium oxide (Al 2 O 3) l aluminium oxide as a base aluminium + hydrochloric acid oxide l aluminium + water chloride aluminium oxide as an acid aluminium sodium + hydroxide sodium + aluminate water

Neutral oxides Non-metals that form oxides that show neither basic nor acidic properties. l Insoluble in water. l
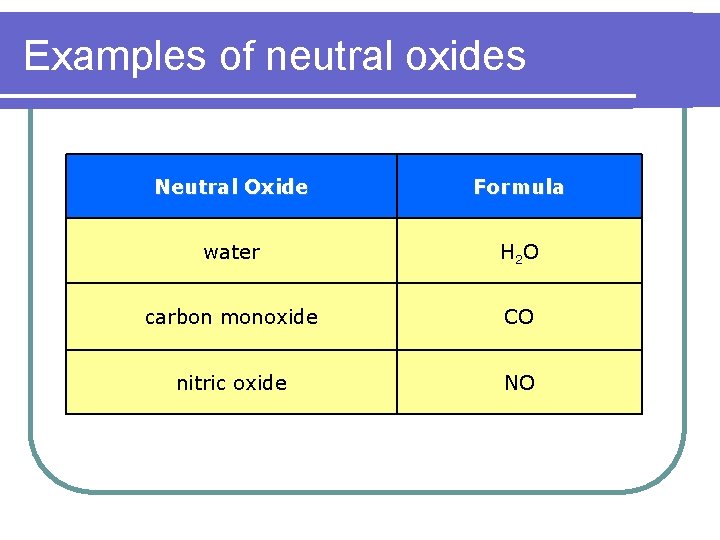
Examples of neutral oxides Neutral Oxide Formula water H 2 O carbon monoxide CO nitric oxide NO

How can we classify an unknown oxide? First question: Does the oxide dissolve in acid? YES NO
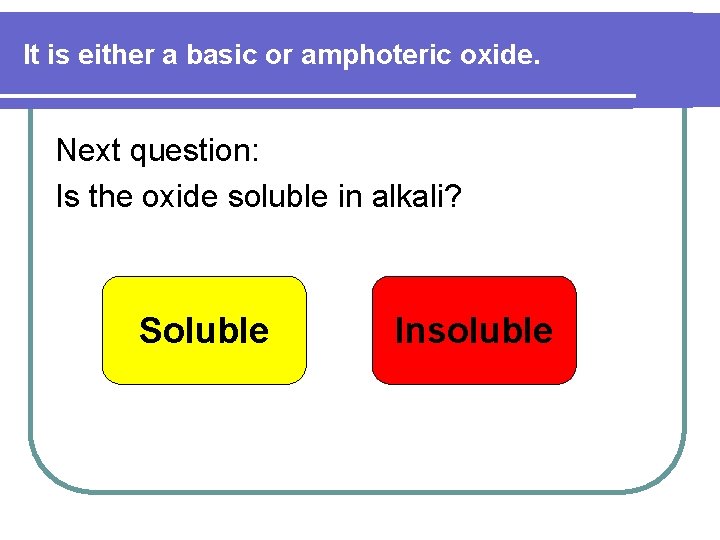
It is either a basic or amphoteric oxide. Next question: Is the oxide soluble in alkali? Soluble Insoluble
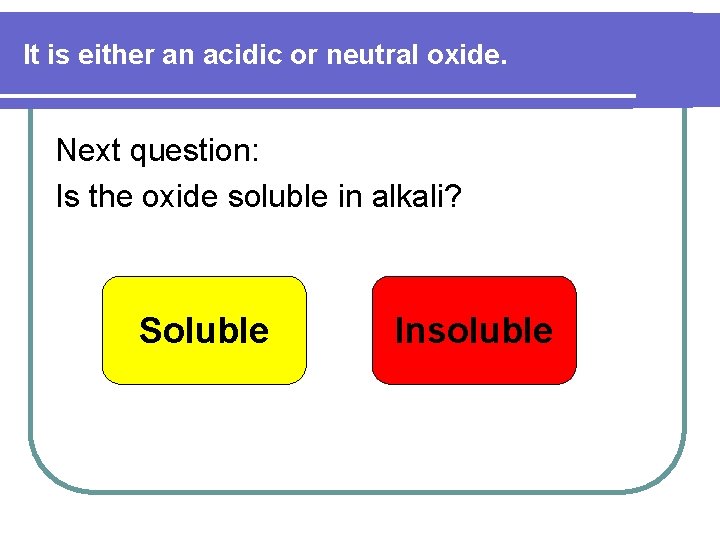
It is either an acidic or neutral oxide. Next question: Is the oxide soluble in alkali? Soluble Insoluble
Amphoteric oxide What is an amphoteric oxide? Back to first question
Basic oxide What is a basic oxide? Cook an egg with calcium oxide Back to first question

Acidic oxide What is an acidic oxide? How does acidic oxide affects the atmosphere? Back to first question
Neutral oxide What is an neutral oxide? Back to first question Back to the first slide
- Slides: 21