Acid and Bases Oxides Many acids and bases
Acid and Bases Oxides Many acids and bases are formed by dissolving oxides in water

At the end of this e-learning, you should able to: l Classify oxides as acidic, basic, amphoteric or neutral based on metallic/non-metallic character

What is an oxide? l An oxide is a compound of oxygen and another element. l Most oxides can be grouped into 2 types: acidic oxides l basic oxides l
Acidic oxides l Oxides of non-metals l Acidic oxides are often gases at room temperature.
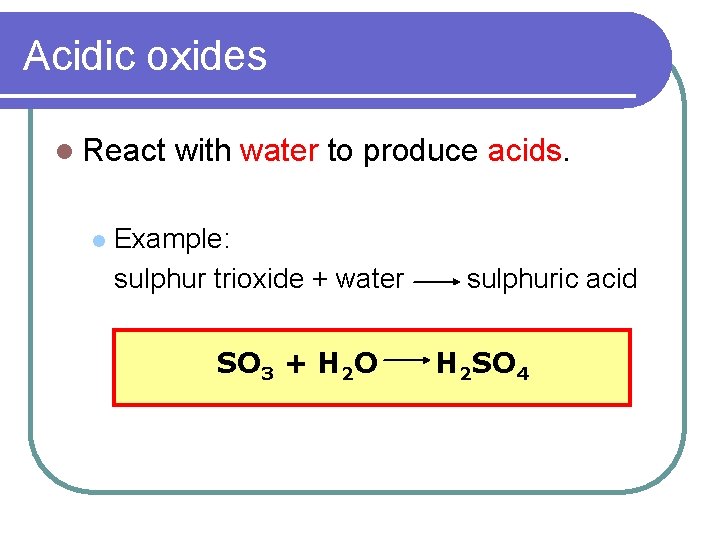
Acidic oxides l React l with water to produce acids. Example: sulphur trioxide + water SO 3 + H 2 O sulphuric acid H 2 SO 4
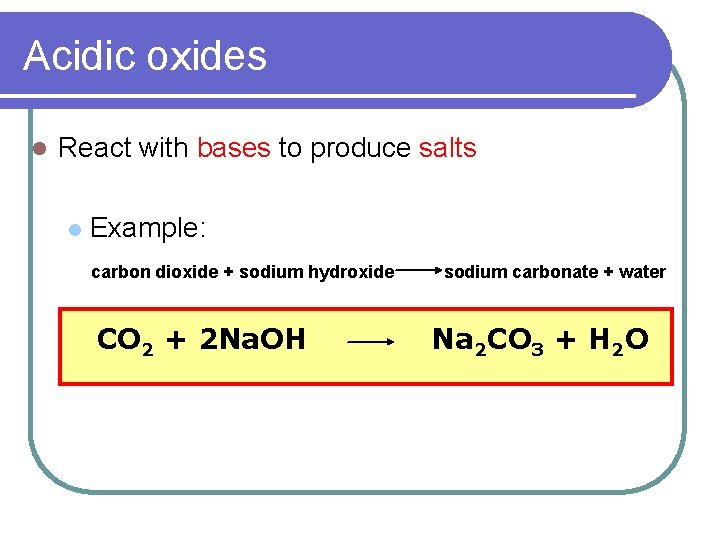
Acidic oxides l React with bases to produce salts l Example: carbon dioxide + sodium hydroxide CO 2 + 2 Na. OH sodium carbonate + water Na 2 CO 3 + H 2 O
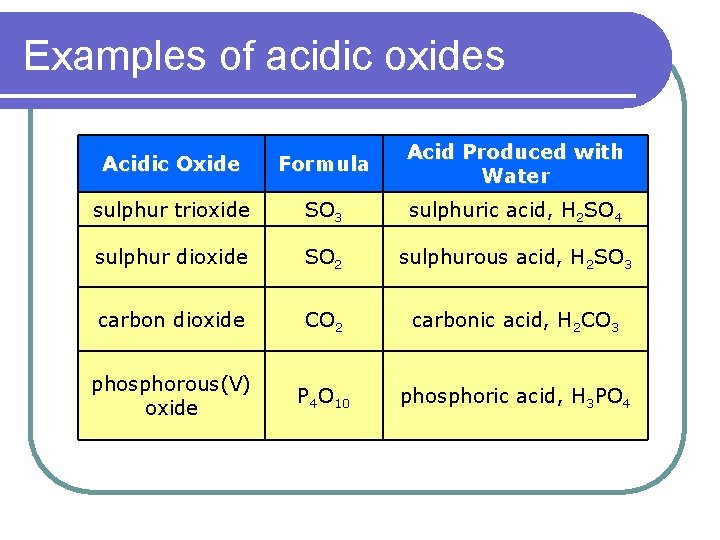
Examples of acidic oxides Acidic Oxide Formula Acid Produced with Water sulphur trioxide SO 3 sulphuric acid, H 2 SO 4 sulphur dioxide SO 2 sulphurous acid, H 2 SO 3 carbon dioxide CO 2 carbonic acid, H 2 CO 3 phosphorous(V) oxide P 4 O 10 phosphoric acid, H 3 PO 4

Basic oxides Oxides of metal l Basic oxides are often solids at room temperature. l Most basic oxides are insoluble in water. l Calcium oxide (quicklime)
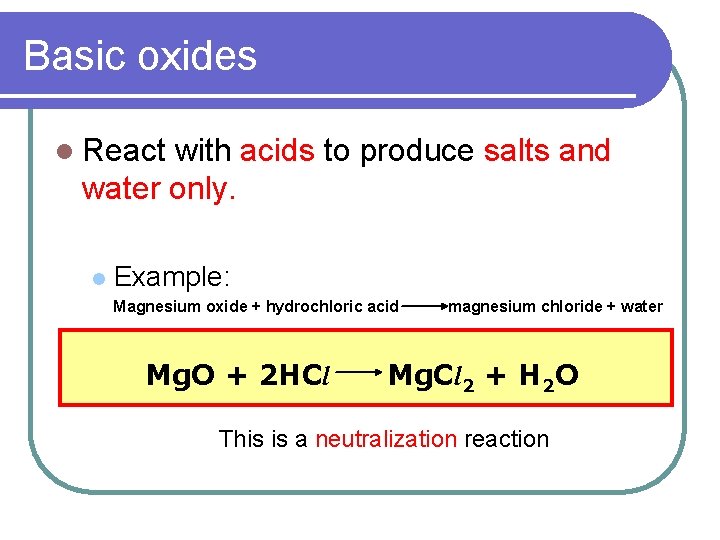
Basic oxides l React with acids to produce salts and water only. l Example: Magnesium oxide + hydrochloric acid Mg. O + 2 HCl magnesium chloride + water Mg. Cl 2 + H 2 O This is a neutralization reaction
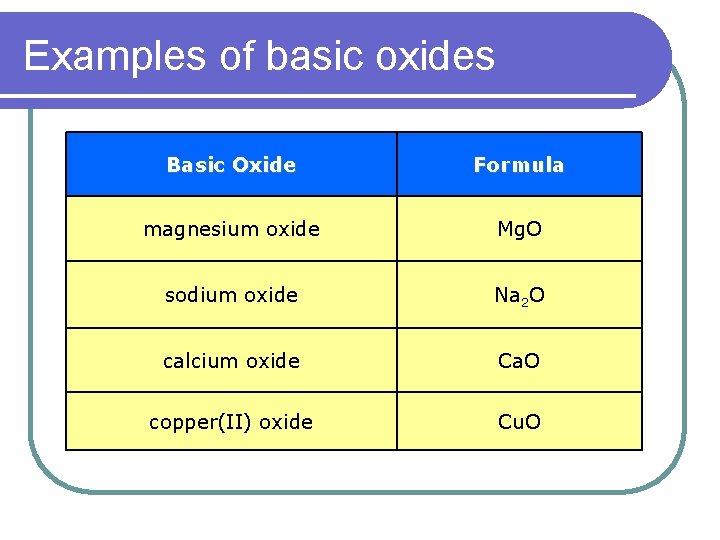
Examples of basic oxides Basic Oxide Formula magnesium oxide Mg. O sodium oxide Na 2 O calcium oxide Ca. O copper(II) oxide Cu. O

Neutral oxides Non-metals that form oxides that show neither basic nor acidic properties. l Insoluble in water. l
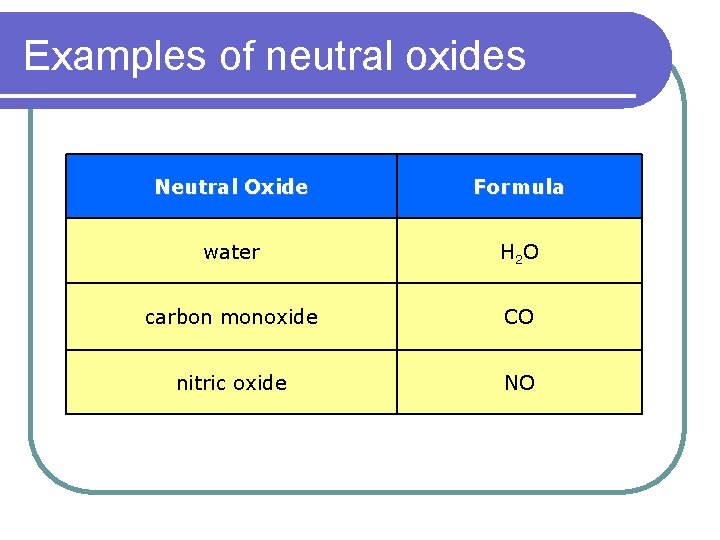
Examples of neutral oxides Neutral Oxide Formula water H 2 O carbon monoxide CO nitric oxide NO
- Slides: 12