Acid and Bases Chapter 24 Examples of Everyday

Acid and Bases Chapter 24
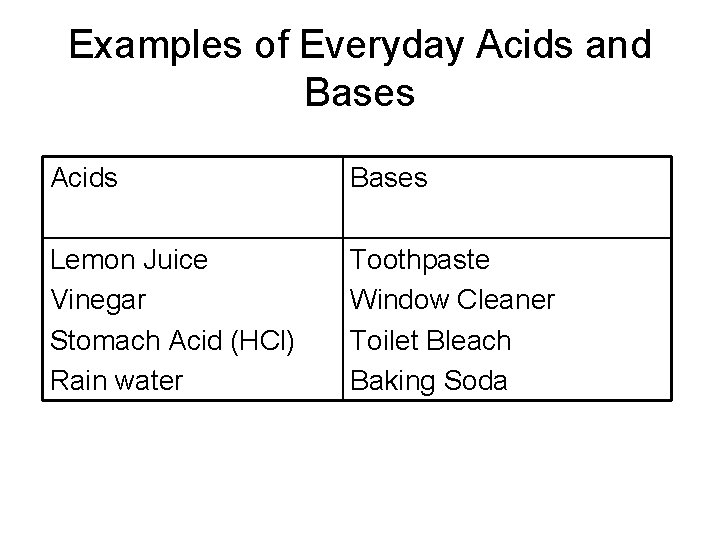
Examples of Everyday Acids and Bases Acids Bases Lemon Juice Vinegar Stomach Acid (HCl) Rain water Toothpaste Window Cleaner Toilet Bleach Baking Soda

Litmus Paper • Litmus paper can be used to tell if something is an acid, base or neutral. Acid Neutral Base Red stays red. Blue turns red. Red stays red. Blue stays blue. Red turns blue.
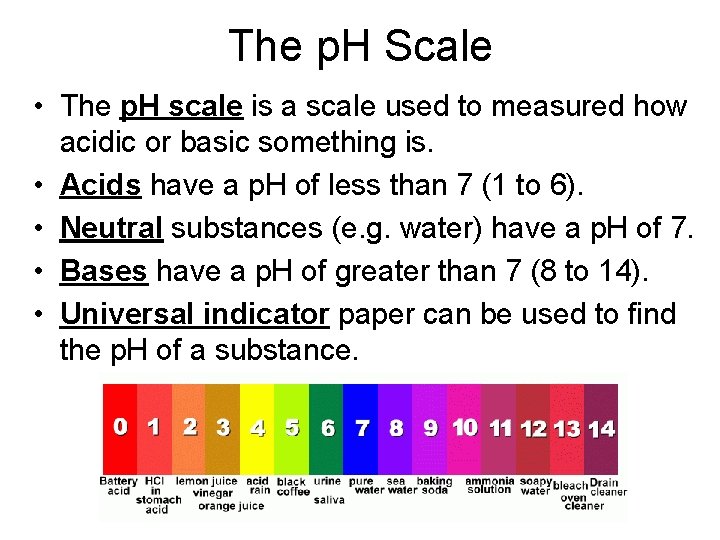
The p. H Scale • The p. H scale is a scale used to measured how acidic or basic something is. • Acids have a p. H of less than 7 (1 to 6). • Neutral substances (e. g. water) have a p. H of 7. • Bases have a p. H of greater than 7 (8 to 14). • Universal indicator paper can be used to find the p. H of a substance.

Alkaline • An alkali is a base which dissolves in water. Example: toothpaste • We say the substance is alkaline.
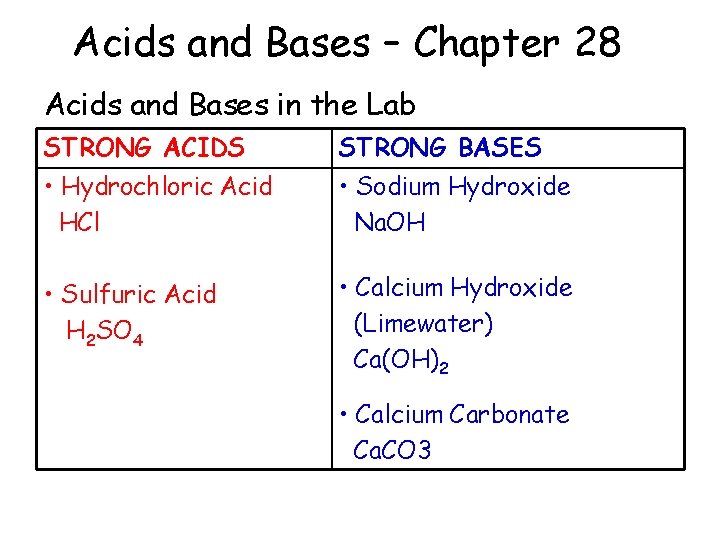
Acids and Bases – Chapter 28 Acids and Bases in the Lab STRONG ACIDS STRONG BASES • Hydrochloric Acid HCl • Sodium Hydroxide Na. OH • Sulfuric Acid H 2 SO 4 • Calcium Hydroxide (Limewater) Ca(OH)2 • Calcium Carbonate Ca. CO 3

Neutralisation occurs when an acid and a base react to make something which is neutral (p. H 7) Examples of neutralisation reactions: § Putting vinegar (acid) on a wasp sting (basic) § Taking antacid tablets (bases) to neutralise excess stomach acid

Indicators Ø To show that neutralisation has occurred we use an indicator. Ø An indicator is any substance which can show whether something is an acid or a base by changing colour. Examples of indicators: § Litmus Paper – red in acid, blue in base § Universal indicator paper - changes colour depending on p. H § Methyl Orange – turns form orange to pink when neutralisation occurs.
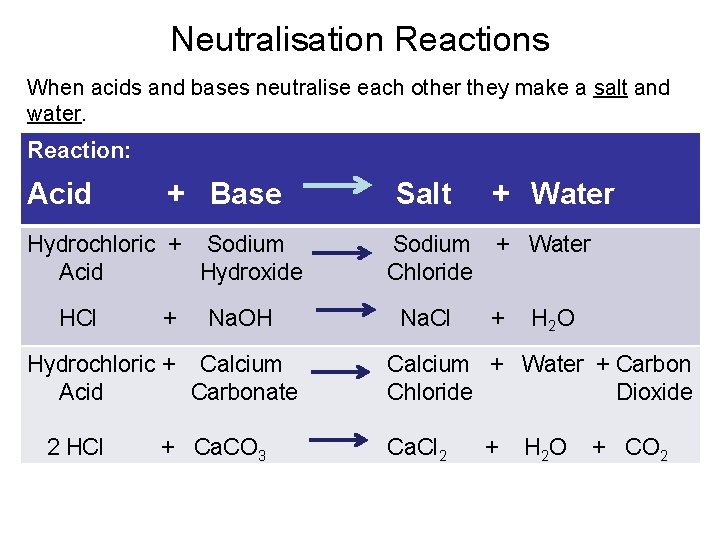
Neutralisation Reactions When acids and bases neutralise each other they make a salt and water. Reaction: Acid + Base Hydrochloric + Sodium Acid Hydroxide HCl + Na. OH Hydrochloric + Calcium Acid Carbonate 2 HCl + Ca. CO 3 Salt Sodium Chloride Na. Cl + Water + H 2 O Calcium + Water + Carbon Chloride Dioxide Ca. Cl 2 + H 2 O + CO 2
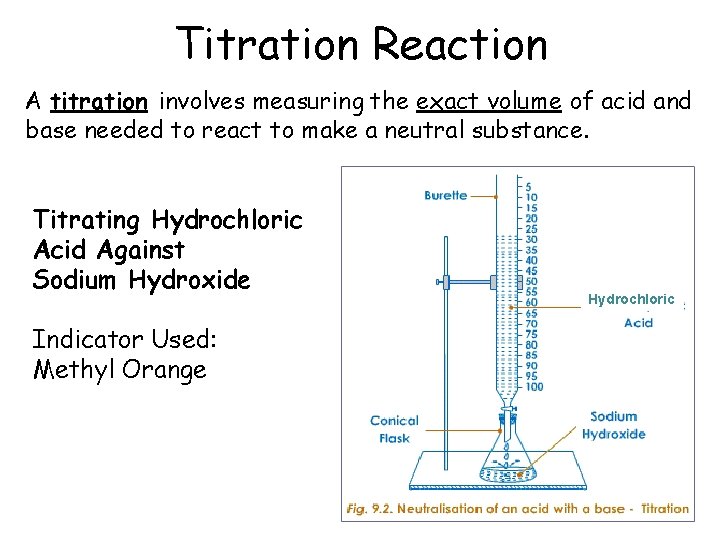
Titration Reaction A titration involves measuring the exact volume of acid and base needed to react to make a neutral substance. Titrating Hydrochloric Acid Against Sodium Hydroxide Indicator Used: Methyl Orange Hydrochloric

What you need to know: Chapter 24 Chapter 28
- Slides: 11