Acid and Bases An Introduction Properties of Acids

Acid and Bases: An Introduction

Properties of Acids 1. 2. 3. 4. 5. Sour taste Can produce H+ (hydrogen) ions (protons) Change the color of litmus from blue to red Reacts with metals such as Zn and Mg to produce H 2 gas. n Ba(s) + H 2 SO 4 Ba. SO 4(aq) + H 2(g) Reacts with carbonates (CO 32 -)to produce CO 2 n 6. Na. HCO 3(s) + CH 3 COOH(aq) Na. CH 3 COO(aq) + H 2 O(l) + CO 2(g) Acids conduct an electric current

Properties of Bases 1. 2. 3. 4. 5. 6. Bitter or caustic taste A slippery, soapy feeling Can produce OH- (hydroxide) ions Ability to change litmus from red to blue Bases conduct an electric current Bases react with acids to produce a salt and water. This is known as a neutralization reaction. n n HCl + Na. OH Na. Cl + H 2 O HCl + Mg(OH)2 Mg. Cl 2 + H 2 O acid base salt water

Ions in Solution n Acidic solutions contains more hydrogen (H+) ions than hydroxide ions (OH-) p. H < 7 Basic solutions contains more hydroxide ions (OH-) than hydrogen ions p. H > 7 Neutral solutions contain an equal concentration of hydrogen and hydroxide ions p. H = 7
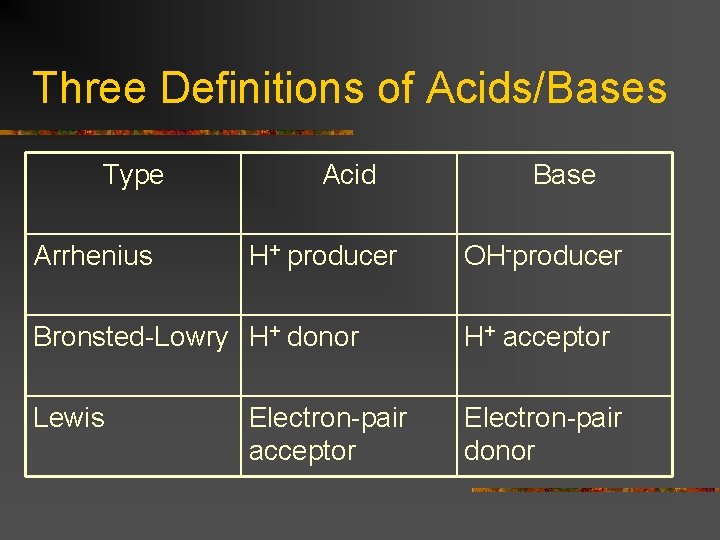
Three Definitions of Acids/Bases Type Arrhenius Acid H+ producer Base OH-producer Bronsted-Lowry H+ donor H+ acceptor Lewis Electron-pair donor Electron-pair acceptor
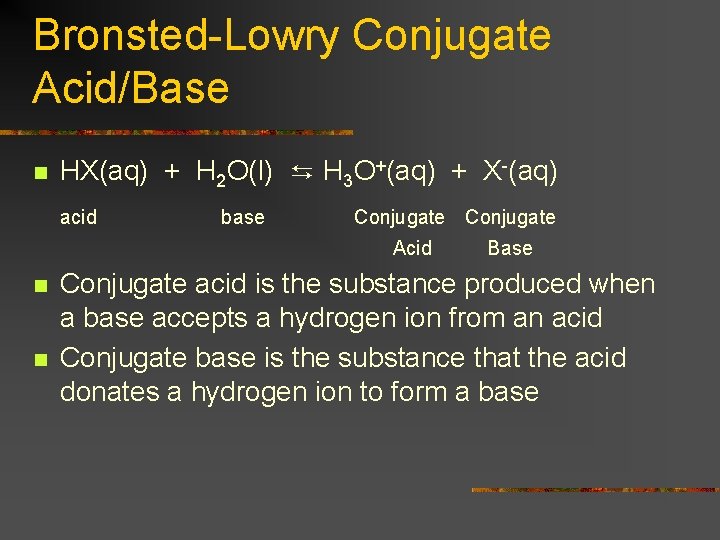
Bronsted-Lowry Conjugate Acid/Base n HX(aq) + H 2 O(l) ⇆ H 3 O+(aq) + X-(aq) acid base Conjugate Acid n n Base Conjugate acid is the substance produced when a base accepts a hydrogen ion from an acid Conjugate base is the substance that the acid donates a hydrogen ion to form a base
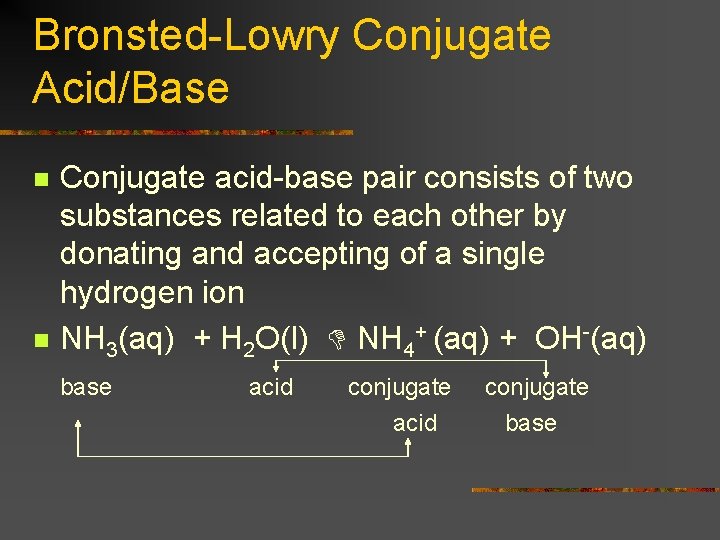
Bronsted-Lowry Conjugate Acid/Base n n Conjugate acid-base pair consists of two substances related to each other by donating and accepting of a single hydrogen ion NH 3(aq) + H 2 O(l) NH 4+ (aq) + OH-(aq) base acid conjugate base
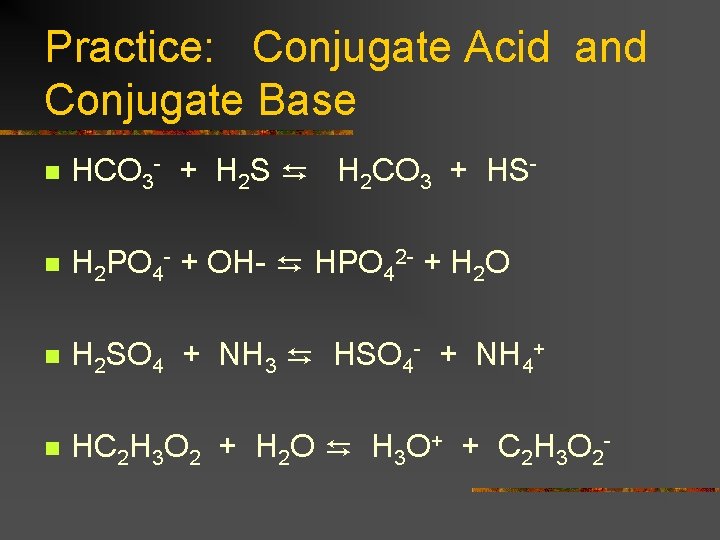
Practice: Conjugate Acid and Conjugate Base n HCO 3 - + H 2 S ⇆ H 2 CO 3 + HS- n H 2 PO 4 - + OH- ⇆ HPO 42 - + H 2 O n H 2 SO 4 + NH 3 ⇆ HSO 4 - + NH 4+ n HC 2 H 3 O 2 + H 2 O ⇆ H 3 O+ + C 2 H 3 O 2 -

Question Time n n Name three models of acids/bases. What is the Arrhenius model of acids/bases? What is the Bronsted-Lowry Model of Acid bases? What is a conjugate acid and a conjugate base?
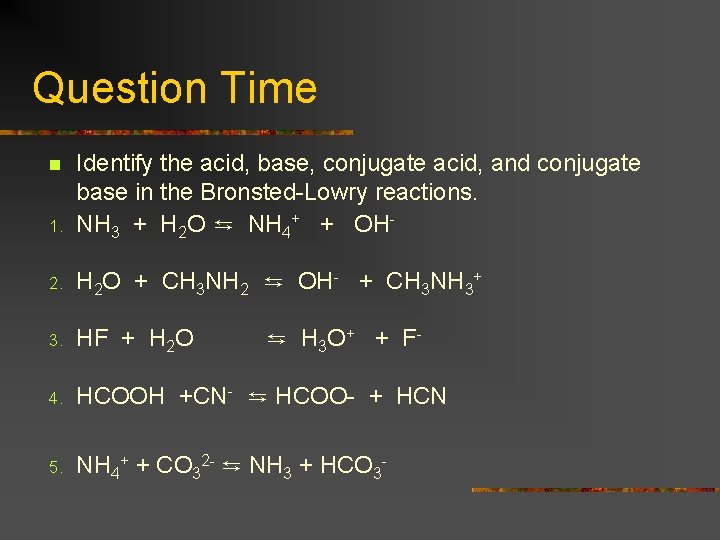
Question Time 1. Identify the acid, base, conjugate acid, and conjugate base in the Bronsted-Lowry reactions. NH 3 + H 2 O ⇆ NH 4+ + OH- 2. H 2 O + CH 3 NH 2 ⇆ OH- + CH 3 NH 3+ 3. HF + H 2 O 4. HCOOH +CN- ⇆ HCOO- + HCN 5. NH 4+ + CO 32 - ⇆ NH 3 + HCO 3 - n ⇆ H 3 O + + F -

Water in Acid/Base solutions n n n Water and other substances can act as both acids and bases and are said to be amphoteric Hydronium ion = H 3 O+ = H+ (Remember H+ is a proton) H 2 O(l) + H 2 O(l) ⇆ H 3 O+(aq) + OH-(aq)

Monoprotic and Polyprotic Acids n n Monoprotic acid donates one hydrogen ion Polyprotic acid donates more than one hydrogen ion

Question Time n n n What is a Lewis acid? What is a Lewis base? What is the H 3 O+ ion called? What is the shorthand way to write H 3 O+? What is amphoteric? Give an example.
- Slides: 13